A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex
- PMID: 10934260
- PMCID: PMC6772566
- DOI: 10.1523/JNEUROSCI.20-16-06106.2000
A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex
Abstract
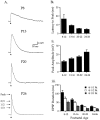
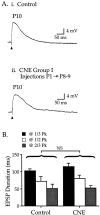
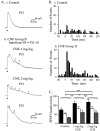
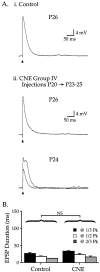
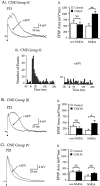
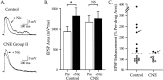
Cholinergic markers in the middle layers of rat auditory cortex are transiently upregulated during the second postnatal week, at which time alpha 7 nicotinic acetylcholine receptors (nAChRs) selectively regulate NMDA receptor (NMDAR)-mediated EPSPs. To investigate the developmental role of this regulation, we determined whether manipulating nAChR function at specific times during the first 4 weeks after birth could alter subsequent neuronal function. Rat pups were injected twice daily with nicotine (1 or 2 mg/kg) or saline during approximately the first, second, or fourth postnatal week (i. e., before, during, or after the peak upregulation of nAChRs). Glutamate EPSPs and intrinsic membrane properties were measured during whole-cell recordings from visually identified pyramidal neurons in layers II-IV of brain slices prepared at least 15 hr after the last injection. Chronic nicotine exposure (CNE) had little effect on intrinsic membrane properties and during week 1 or 4 did not affect synaptic function. However, CNE during week 2 resulted in EPSPs with long durations, multiple peaks, and enhanced NMDAR components. These changes remained significant even 10 d after CNE. Rapid application of nicotine, which in control neurons selectively enhances NMDAR EPSPs during week 2, produced only weak effects after CNE. Receptor binding studies showed that CNE-induced EPSP alterations occurred in the absence of altered alpha 7 nAChR numbers or agonist binding affinity. Thus, altered stimulation of nAChRs by CNE during week 2, but not before or after, disrupts the development of glutamate synapses in rat auditory cortex.
Figures

Similar articles
-
Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex.J Neurosci. 1998 Oct 15;18(20):8485-95. doi: 10.1523/JNEUROSCI.18-20-08485.1998. J Neurosci. 1998. PMID: 9763491 Free PMC article.
-
Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain.Brain Res Dev Brain Res. 2002 Jan 31;133(1):19-25. doi: 10.1016/s0165-3806(01)00314-5. Brain Res Dev Brain Res. 2002. PMID: 11850060
-
Synaptic potentials mediated by alpha 7 nicotinic acetylcholine receptors in supraoptic nucleus.J Neurosci. 2002 Jan 1;22(1):29-37. doi: 10.1523/JNEUROSCI.22-01-00029.2002. J Neurosci. 2002. PMID: 11756485 Free PMC article.
-
Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats.Eur J Neurosci. 2006 Aug;24(3):857-66. doi: 10.1111/j.1460-9568.2006.04945.x. Epub 2006 Jul 18. Eur J Neurosci. 2006. PMID: 16848798
-
Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing.Respir Physiol Neurobiol. 2008 Dec 10;164(1-2):80-6. doi: 10.1016/j.resp.2008.05.008. Respir Physiol Neurobiol. 2008. PMID: 18585984 Free PMC article. Review.
Cited by
-
Alpha7 nicotinic acetylcholine receptors occur at postsynaptic densities of AMPA receptor-positive and -negative excitatory synapses in rat sensory cortex.J Neurosci. 2002 Jun 15;22(12):5001-15. doi: 10.1523/JNEUROSCI.22-12-05001.2002. J Neurosci. 2002. PMID: 12077196 Free PMC article.
-
Chronic neonatal nicotine exposure increases excitation in the young adult rat hippocampus in a sex-dependent manner.Brain Res. 2012 Jan 9;1430:8-17. doi: 10.1016/j.brainres.2011.10.039. Epub 2011 Nov 4. Brain Res. 2012. PMID: 22119395 Free PMC article.
-
Nicotinic alteration of functional thalamocortical topography.Neuroreport. 2015 Aug 19;26(12):688-94. doi: 10.1097/WNR.0000000000000409. Neuroreport. 2015. PMID: 26164456 Free PMC article.
-
Unique effects of nicotine across the lifespan.Pharmacol Biochem Behav. 2022 Mar;214:173343. doi: 10.1016/j.pbb.2022.173343. Epub 2022 Feb 3. Pharmacol Biochem Behav. 2022. PMID: 35122768 Free PMC article. Review.
-
Prenatal Nicotine Exposure Disrupts Infant Neural Markers of Orienting.Nicotine Tob Res. 2018 Jun 7;20(7):897-902. doi: 10.1093/ntr/ntx177. Nicotine Tob Res. 2018. PMID: 29059450 Free PMC article.
References
-
- Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, Sinden JD. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology (Berl) 1996;124:323–331. - PubMed
-
- Agmon A, O'Dowd DK. NMDA receptor-mediated currents are prominent in the thalamocortical synaptic response before maturation of inhibition. J Neurophysiol. 1992;68:345–349. - PubMed
-
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. - PubMed
-
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
