Postsynaptic variability of firing in rat cortical neurons: the roles of input synchronization and synaptic NMDA receptor conductance
- PMID: 10934268
- PMCID: PMC6772582
- DOI: 10.1523/JNEUROSCI.20-16-06181.2000
Postsynaptic variability of firing in rat cortical neurons: the roles of input synchronization and synaptic NMDA receptor conductance
Abstract
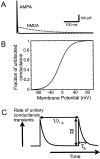
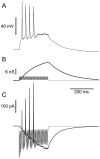
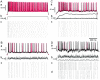
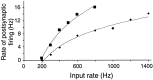
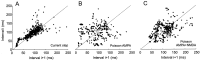
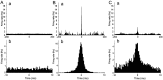
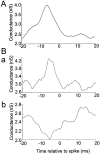
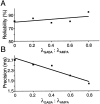
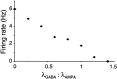
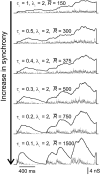
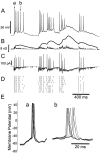
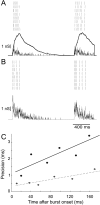
Neurons in the functioning cortex fire erratically, with highly variable intervals between spikes. How much irregularity comes from the process of postsynaptic integration and how much from fluctuations in synaptic input? We have addressed these questions by recording the firing of neurons in slices of rat visual cortex in which synaptic receptors are blocked pharmacologically, while injecting controlled trains of unitary conductance transients, to electrically mimic natural synaptic input. Stimulation with a Poisson train of fast excitatory (AMPA-type) conductance transients, to simulate independent inputs, produced much less variability than encountered in vivo. Addition of NMDA-type conductance to each unitary event regularized the firing but lowered the precision and reliability of spikes in repeated responses. Independent Poisson trains of GABA-type conductance transients (reversing at the resting potential), which simulated independent activity in a population of presynaptic inhibitory neurons, failed to increase timing variability substantially but increased the precision of responses. However, introduction of synchrony, or correlations, in the excitatory input, according to a nonstationary Poisson model, dramatically raised timing variability to in vivo levels. The NMDA phase of compound AMPA-NMDA events conferred a time-dependent postsynaptic variability, whereby the reliability and precision of spikes degraded rapidly over the 100 msec after the start of a synchronous input burst. We conclude that postsynaptic mechanisms add significant variability to cortical responses but that substantial synchrony of inputs is necessary to explain in vivo variability. We suggest that NMDA receptors help to implement a switch from precise firing to random firing during responses to concerted inputs.
Figures

References
-
- Abeles M. Corticonics: neural circuits of the cerebral cortex. Cambridge UP; Cambridge, UK: 1991.
-
- Aihara K, Matsumoto G. Chaos (Holden AV, ed. Manchester UP; Manchester, UK: 1986. Chaotic oscillations and bifurcations in squid giant axons. pp. 257–269.
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked-cortical responses. Science. 1996;273:1868–1871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
