Causes and effects of heterogeneous perfusion in tumors
- PMID: 10935474
- PMCID: PMC1508079
- DOI: 10.1038/sj.neo.7900037
Causes and effects of heterogeneous perfusion in tumors
Abstract
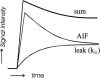
A characteristic of solid tumors is their heterogeneous distribution of blood flow, with significant hypoxia and acidity in low-flow regions. We review effects of heterogeneous tumor perfusion are reviewed and propose a conceptual model for its cause. Hypoxic-acidic regions are resistant to chemo- and radiotherapy and may stimulate progression to a more metastatic phenotype. In normal tissues, hypoxia and acidity induce angiogenesis, which is expected to improve perfusion. However, aggressive tumors can have high local microvessel density simultaneously with significant regions of hypoxia and acidosis. A possible explanation for this apparent contradiction is that the mechanisms regulating growth and adaptation of vascular networks are impaired. According to a recent theory for structural adaptation of vascular networks, four interrelated adaptive responses can work as a self-regulating system to produce a mature and efficient blood distribution system in normal tissues. It is proposed that heterogeneous perfusion in tumors may result from perturbation of this system. Angiogenesis may increase perfusion heterogeneity in tumors by increasing the disparity between parallel low- and high-resistance flow pathways. This conceptual model provides a basis for future rational therapies. For example, it indicates that selective destruction of tumor vasculature may increase perfusion efficiency and improve therapeutic efficacy.
Figures



References
-
- Gullino PM. Tumor pathophysiology: the perfusion model. Antibiot Chemother. 1980;28:35–42. - PubMed
-
- Jain RK. The Eugene M. Landis Award Lecture 1996. Delivery of molecular and cellular medicine to solid tumors. Microcirculation. 1997;4:1–23. - PubMed
-
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen consumption and tissue oxygenation of human tumors. Adv Exp Med Biol. 1990;277:895–905. - PubMed
-
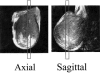
- Kidney DD, Dietrich RB, Goyal AK, Yan K, Bradley WG., Jr MRI of extracranial masses in children: the usefulness of gadolinium-chelate enhancement. Pediatr Radiol. 1998;28:322–328. - PubMed
-
- Furman-Haran E, Grobgeld D, Degani H. Dynamic contrast-enhanced imaging and analysis at high spatial resolution of MCF7 human breast tumors. J Magn Reson. 1997;128:161–171. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
