"...those left behind." Biology and oncology of invasive glioma cells
- PMID: 10935475
- PMCID: PMC1508082
- DOI: 10.1038/sj.neo.7900034
"...those left behind." Biology and oncology of invasive glioma cells
Abstract
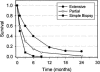
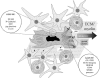
Although significant technical advances in surgical and radiation treatment for brain tumors have emerged in recent years, their impact on clinical outcome for patients has been disappointing. A fundamental source of the management challenge presented by glioma patients is the insidious propensity of the malignant cells to invade into adjacent normal brain. Invasive tumor cells escape surgical removal and geographically dodge lethal radiation exposure. Recent improved understanding of the biochemistry and molecular determinants of glioma cell invasion provide valuable insight to the underlying biological features of the disease, as well as illuminating possible new therapeutic targets. Heightened commitment to migrate and invade is accompanied by a glioma cell's reduced proliferative activity. The microenvironmental manipulations coincident to invasion and migration may also impact the glioma cell's response to cytotoxic treatments. These collateral aspects of the glioma cell invasive phenotype should be further explored and exploited as novel antiglioma therapies.
Figures




References
-
- 1997 Annual Report. Central Brain Tumor Registry of the United States Chicago, IL. 1998:1.
-
- Burger PC, Dubois PJ, Schold SC, Smith KR, Odom GL, Crafts DC, Giangaspero F. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983;58:159–169. - PubMed
-
- Gaspar LE, Fisher BJ, MacDonald DR, LeBer DV, Halperin EC, Schold SC, Cairncross JG. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24:55–57. - PubMed
-
- Brem H, Ewend MG, Piantadosi S, Greenhoot J, Burger PC, Sisti M. The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: Phase I trial. J Neurooncol. 1995;26:111–123. - PubMed
-
- Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, Long Z, Chiang Y, McGarrity GJ, Muul LM, Katz D, Blaese RM, Oldfield EH. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells [see comments] Nat Med. 1997;3:1354–1361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
