Dynamic remodeling of the vascular bed precedes tumor growth: MLS ovarian carcinoma spheroids implanted in nude mice
- PMID: 10935477
- PMCID: PMC1508074
- DOI: 10.1038/sj.neo.7900032
Dynamic remodeling of the vascular bed precedes tumor growth: MLS ovarian carcinoma spheroids implanted in nude mice
Abstract
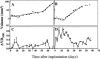
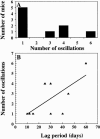
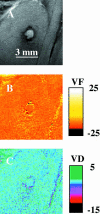
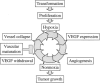
The goal of this study was to monitor the vascular bed during the lag phase in growth of implanted spheroids as a model of tumor dormancy. Vascular development and tumor growth were followed up by magnetic resonance imaging in a model system of MLS ovarian carcinoma spheroids implanted subcutaneously in female nude mice. Apparent vessel density in a 1-mm rim surrounding the spheroid was evaluated by gradient echo imaging as a measure of the angiogenic potential of the tumor. Vascular functionality and maturation were assessed by signal intensity changes in response to hyperoxia (elevated oxygen) and hypercapnia (elevated carbon dioxide), respectively. Tumor growth was delayed by 12 to 57 days after implantation. During this long period in which tumor volume did not change, up to 6 cycles of vascular development and regression were observed. We propose here that dynamic remodeling of the vascular bed may precede exit of tumors from dormancy. The sustained oscillations in the angiogenic response to the implanted spheroid are consistent with hypoxic regulation of vascular endothelial growth factor (VEGF), combined with the role of VEGF as an essential survival factor for newly formed blood vessels. Vascular maturation, manifested by physiological vasodilatory response to carbon dioxide, may be important for conferring vascular stability and exit from dormancy.
Figures
References
-
- Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328:1237–1243. - PubMed
-
- Marches R, Scheuermann RH, Uhr JW. Cancer dormancy: role of cyclin-dependent kinase inhibitors in induction of cell cycle arrest mediated via membrane IgM. Cancer Res. 1998;58:691–697. - PubMed
-
- Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56:5754–5757. - PubMed
-
- Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. - PubMed
-
- Stewart TH. Immune mechanisms and tumor dormancy. Medicina (B Aires) 1996;56(suppl 1):74–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
