Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in human transitional bladder cancer and its role in inducing cell death
- PMID: 10935488
- PMCID: PMC1508103
- DOI: 10.1038/sj.neo.7900050
Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in human transitional bladder cancer and its role in inducing cell death
Abstract
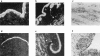
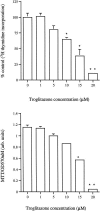
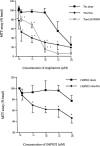
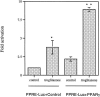
The present study examined the expression and role of the thiazolidinedione (TZD)-activated transcription factor, peroxisome proliferator-activated receptor gamma (PPARgamma), in human bladder cancers. In situ hybridization shows that PPARgamma mRNA is highly expressed in all human transitional epithelial cell cancers (TCCa's) studied (n=11). PPARgamma was also expressed in five TCCa cell lines as determined by RNase protection assays and immunoblot. Retinoid X receptor alpha (RXRalpha), a 9-cis-retinoic acid stimulated (9-cis-RA) heterodimeric partner of PPARgamma, was also co-expressed in all TCCa tissues and cell lines. Treatment of the T24 bladder cancer cells with the TZD PPARgamma agonist troglitazone, dramatically inhibited 3H-thymidine incorporation and induced cell death. Addition of the RXRalpha ligands, 9-cis-RA or LG100268, sensitized T24 bladder cancer cells to the lethal effect of troglitazone and two other PPAR- activators, ciglitazone and 15-deoxy-delta(12,14)-PGJ2 (15dPGJ(2)). Troglitazone treatment increased expression of two cyclin-dependent kinase inhibitors, p21(WAF1/CIP1) and p16(INK4), and reduced cyclin D1 expression, consistent with G1 arrest. Troglitazone also induced an endogenous PPARgamma target gene in T24 cells, adipocyte-type fatty acid binding protein (A-FABP), the expression of which correlates with bladder cancer differentiation. In situ hybridization shows that A-FABP expression is localized to normal uroepithelial cells as well as some TCCa's. Taken together, these results demonstrate that PPARgamma is expressed in human TCCa where it may play a role in regulating TCCa differentiation and survival, thereby providing a potential target for therapy of uroepithelial cancers.
Figures


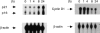

Similar articles
-
Peroxisome proliferator-activated receptor gamma reduces the growth rate of pancreatic cancer cells through the reduction of cyclin D1.Life Sci. 2002 Feb 15;70(13):1565-75. doi: 10.1016/s0024-3205(01)01524-7. Life Sci. 2002. PMID: 11895107
-
A novel method for analysis of nuclear receptor function at natural promoters: peroxisome proliferator-activated receptor gamma agonist actions on aP2 gene expression detected using branched DNA messenger RNA quantitation.Mol Endocrinol. 1999 Mar;13(3):410-7. doi: 10.1210/mend.13.3.0246. Mol Endocrinol. 1999. PMID: 10076998
-
Thiazolidinediones inhibit growth of gastrointestinal, biliary, and pancreatic adenocarcinoma cells through activation of the peroxisome proliferator-activated receptor gamma/retinoid X receptor alpha pathway.Exp Cell Res. 2003 Sep 10;289(1):143-51. doi: 10.1016/s0014-4827(03)00263-5. Exp Cell Res. 2003. PMID: 12941612
-
Peroxisome proliferator-activated receptor gamma (PPargamma) as a novel target for prostate cancer.Invest New Drugs. 2002 May;20(2):195-200. doi: 10.1023/a:1015670126203. Invest New Drugs. 2002. PMID: 12099579 Review.
-
Complexity, retinoid-responsive gene networks, and bladder carcinogenesis.Adv Exp Med Biol. 1999;462:449-67. doi: 10.1007/978-1-4615-4737-2_35. Adv Exp Med Biol. 1999. PMID: 10599447 Review.
Cited by
-
The Role of PPARs in Cancer.PPAR Res. 2008;2008:102737. doi: 10.1155/2008/102737. PPAR Res. 2008. PMID: 18584037 Free PMC article.
-
PPARgamma and Apoptosis in Cancer.PPAR Res. 2008;2008:704165. doi: 10.1155/2008/704165. PPAR Res. 2008. PMID: 18615184 Free PMC article.
-
Pioglitazone and bladder cancer risk: a systematic review and meta-analysis.Cancer Med. 2018 Apr;7(4):1070-1080. doi: 10.1002/cam4.1354. Epub 2018 Feb 24. Cancer Med. 2018. PMID: 29476615 Free PMC article.
-
Peroxisome Proliferator-Activated Receptors and Caloric Restriction-Common Pathways Affecting Metabolism, Health, and Longevity.Cells. 2020 Jul 16;9(7):1708. doi: 10.3390/cells9071708. Cells. 2020. PMID: 32708786 Free PMC article. Review.
-
Expression of peroxisome proliferator-activated receptor-gamma in colon cancer: correlation with histopathological parameters, cell cycle-related molecules, and patients' survival.Dig Dis Sci. 2007 Sep;52(9):2305-11. doi: 10.1007/s10620-007-9794-4. Epub 2007 Mar 28. Dig Dis Sci. 2007. PMID: 17393321
References
-
- Brun RP, Tontonoz P, Forman BM, Ellis R, Chen J, Evans RM, Spiegelman BM. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996;10:974–984. - PubMed
-
- Guan Y, Zhang Y, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am J Physiol. 1997;273:F1013–F1022. - PubMed
-
- Jiang C, Ting AT, Seed B. PPARg agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. - PubMed
-
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors a and g are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Blol Chem. 1997;272:3406–3410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
