Novel suicide ligands of tubulin arrest cancer cells in S-phase
- PMID: 10935497
- PMCID: PMC1508119
- DOI: 10.1038/sj.neo.7900066
Novel suicide ligands of tubulin arrest cancer cells in S-phase
Abstract
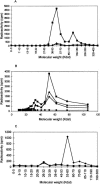
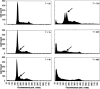
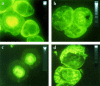
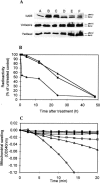
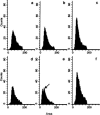
It is presently accepted that the mechanism of action for all anti-tumor tubulin ligands involves the perturbation of microtubule dynamics during the G2/M phase of cell division and subsequent entry into apoptosis [1]. In this report, we challenge the established dogma by describing a unique mechanism of action caused by a novel series of tubulin ligands, halogenated derivatives of acetamido benzoyl ethyl ester. We have developed a suicide ligand for tubulin, which covalently attaches to the target and shows potent cancericidal activity in tissue culture assays and in animal tumor models. These compounds target early S-phase at the G1/S transition rather than the G2/M phase and mitotic arrest. Bcl-2 phosphorylation, a marker of mitotic microtubule inhibition by other tubulin ligands was dramatically altered, phosphorylation was rapid and biphasic rather than a slow linear event. The halogenated ethyl ester series of derivatives thus constitute a unique set of tubulin ligands which induce a novel mechanism of apoptosis.
Figures
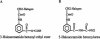
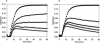
Similar articles
-
Double blockade of cell cycle at g(1)-s transition and m phase by 3-iodoacetamido benzoyl ethyl ester, a new type of tubulin ligand.Cancer Res. 2002 Nov 1;62(21):6080-8. Cancer Res. 2002. PMID: 12414632
-
3-(Iodoacetamido)-benzoylurea: a novel cancericidal tubulin ligand that inhibits microtubule polymerization, phosphorylates bcl-2, and induces apoptosis in tumor cells.Cancer Res. 1998 Dec 1;58(23):5389-95. Cancer Res. 1998. PMID: 9850070
-
Novel Benzo[B]Furans with Anti-Microtubule Activity Upregulate Expression of Apoptotic Genes and Arrest Leukemia Cells in G2/M Phase.Anticancer Agents Med Chem. 2019;19(3):375-388. doi: 10.2174/1871520619666181122123552. Anticancer Agents Med Chem. 2019. PMID: 30465514
-
Investigation of anti-tumor mechanisms of K2154: characterization of tubulin isotypes, mitotic arrest and apoptotic machinery.Naunyn Schmiedebergs Arch Pharmacol. 2006 Dec;374(3):223-33. doi: 10.1007/s00210-006-0114-x. Epub 2006 Nov 11. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 17102938
-
An overview on anti-tubulin agents for the treatment of lymphoma patients.Pharmacol Ther. 2020 Jul;211:107552. doi: 10.1016/j.pharmthera.2020.107552. Epub 2020 Apr 17. Pharmacol Ther. 2020. PMID: 32305312 Review.
Cited by
-
M3, a natural lignan xyloside, exhibits potent anticancer activity in HCT116 cells.Oncol Lett. 2019 Feb;17(2):2117-2122. doi: 10.3892/ol.2018.9823. Epub 2018 Dec 12. Oncol Lett. 2019. PMID: 30675278 Free PMC article.
-
Membrane-active host defense peptides--challenges and perspectives for the development of novel anticancer drugs.Chem Phys Lipids. 2011 Nov;164(8):766-81. doi: 10.1016/j.chemphyslip.2011.09.004. Epub 2011 Sep 16. Chem Phys Lipids. 2011. PMID: 21945565 Free PMC article. Review.
-
A 4-Phenoxyphenol Derivative Exerts Inhibitory Effects on Human Hepatocellular Carcinoma Cells through Regulating Autophagy and Apoptosis Accompanied by Downregulating α-Tubulin Expression.Molecules. 2017 May 21;22(5):854. doi: 10.3390/molecules22050854. Molecules. 2017. PMID: 28531143 Free PMC article.
-
The effect of beta-elemene on alpha-tubulin polymerization in human hepatoma HepG2 cells.Chin J Cancer Res. 2013 Dec;25(6):770-6. doi: 10.3978/j.issn.1000-9604.2013.12.12. Chin J Cancer Res. 2013. PMID: 24385707 Free PMC article.
-
Ampelopsin-sodium induces apoptosis in human lung adenocarcinoma cell lines by promoting tubulin polymerization in vitro.Oncol Lett. 2019 Jul;18(1):189-196. doi: 10.3892/ol.2019.10288. Epub 2019 Apr 30. Oncol Lett. 2019. PMID: 31289488 Free PMC article.
References
-
- Dumontet C, Sikic B. Mechanisms of action of and resistance to anti-tubulin agents: microtubule dynamics, drug transport and cell death. Review in J Clin Oncol. 1999;17:1061–1070. - PubMed
-
- Rowinsky EK, Donehower RC. The clinical pharmacology and use of anti-microtubule agents in cancer chemotherapeutics. Pharmacol Ther. 1991;52:35–84. - PubMed
-
- Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970;9:4999–5007. - PubMed
-
- Wilson L, Creswell CR, Chin D. The mechanism of action of vinblastine. Binding of [acetyl-3H] vinblastine to embryonic chick brain tubulin and tubulin from sea urchin sperm tail outer doublet microtubules. Biochemistry. 1975;14:5586–5592. - PubMed
-
- Malawista SE, Bensch KG, Sato H. Vinblastine and griseofulvin reversibly disrupt the living mitotic spindle. Science. 1968;160:770–772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
