A bcl-xS adenovirus selectively induces apoptosis in transformed cells compared to normal mammary cells
- PMID: 10935511
- PMCID: PMC1507566
- DOI: 10.1038/sj.neo.7900084
A bcl-xS adenovirus selectively induces apoptosis in transformed cells compared to normal mammary cells
Abstract
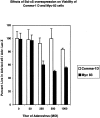
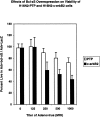
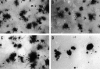
Oncogenes which drive the cell cycle, such as c-myc, can sensitize cells to apoptosis. This suggests the possibility that the expression of genes such as bcl-2 or bcl-xL is required to inhibit apoptosis induced by oncogene expression. We hypothesized that inhibition of Bcl-2/Bcl-xL by the pro-apoptotic Bcl-xS protein, would result in selective induction of apoptosis in mammary carcinoma cells compared to their nontransformed counterparts. Therefore, we compared the effects of Bcl-xS expression delivered by a bcl-xS adenovirus (bcl-xS-Adv) vector, on viability and apoptosis of nontransformed versus transformed mammary epithelial cells. We report that c-myc-transformed murine mammary cells are extremely sensitive to apoptosis induced by the bcl-xS adenovirus (bcl-xS-Adv) vector, whereas immortalized, nontransformed murine mammary cells are relatively resistant to apoptosis induced by this vector. Likewise, human mammary epithelial cells transduced with c-erbB-2 were more sensitive to apoptosis induced by the bcl-xS vector than the nontransformed parental cells. Similar results were obtained when we tested the effects of bcl-xS adenoviral infection on primary normal human mammary epithelial cells and SUM-190 PT cells, (a c-erbB-2 over-expressing human mammary carcinoma cell line) grown on Matrigel. These data are consistent with the hypothesis that inhibition of Bcl-2/Bcl-xL can result in selective killing of cancer cells compared to their nontransformed counterparts.
Figures
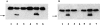

Similar articles
-
bcl-xs gene therapy induces apoptosis of human mammary tumors in nude mice.Cancer Res. 1996 May 1;56(9):1965-9. Cancer Res. 1996. PMID: 8616832
-
Bax and Bcl-xs are induced at the onset of apoptosis in involuting mammary epithelial cells.Mech Dev. 1996 May;56(1-2):197-207. doi: 10.1016/0925-4773(96)88032-4. Mech Dev. 1996. PMID: 8798158
-
Bcl-xS enhances adenoviral vector-induced apoptosis in neuroblastoma cells.Cancer Res. 1996 Dec 15;56(24):5734-40. Cancer Res. 1996. PMID: 8971184
-
Targeting cancer cell death with a bcl-XS adenovirus.Springer Semin Immunopathol. 1998;19(3):279-88. doi: 10.1007/BF00787225. Springer Semin Immunopathol. 1998. PMID: 9580270 Review.
-
Cell cycle checkpoints and apoptosis: potential for improving radiation therapy.Vitam Horm. 1997;53:1-25. doi: 10.1016/s0083-6729(08)60702-5. Vitam Horm. 1997. PMID: 9197176 Review. No abstract available.
Cited by
-
Regulation of Bcl-xL expression in lung vascular smooth muscle.Am J Respir Cell Mol Biol. 2007 Jun;36(6):678-87. doi: 10.1165/rcmb.2006-0359OC. Epub 2007 Feb 1. Am J Respir Cell Mol Biol. 2007. PMID: 17272823 Free PMC article.
References
-
- Krajewski S, Krajewska M, Shabaik A, Wang H, Irie S, Fong L, Reed JC. Immunohistochemical analysis of in vivo patterns of Bcl-x expression. Cancer Res. 1994;54:5501–5507. - PubMed
-
- Olufunmilayo I, Olopade MO, Adenyaju SAR, Hagos M, Mick R, Thompson CB. Overexpression of Bcl-X in primary breast cancer is associated with high tumor grade and metastases. The Cancer Journal from Scientific American. 1997;3:230–237. - PubMed
-
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2 related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. - PubMed
-
- Sumantran VN, Ealovega MW, Nunez G, Clarke MF, Wicha MS. Overexpression of Bcl-xS sensitizes MCF-7 cells to chemotherapy induced apoptosis. Cancer Res. 1995;55:2507–2510. - PubMed
-
- Ealovega MW, McGinnis PK, Sumantran VN, Clarke MF, Wicha MS. bcl-xS gene therapy induces apoptosis of human mammary tumors in Nude mice. Cancer Res. 1996;56:1965–1969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
