The poly(A)-binding protein and an mRNA stability protein jointly regulate an endoribonuclease activity
- PMID: 10938110
- PMCID: PMC86108
- DOI: 10.1128/MCB.20.17.6334-6341.2000
The poly(A)-binding protein and an mRNA stability protein jointly regulate an endoribonuclease activity
Abstract
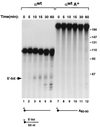
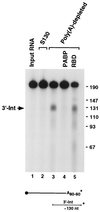
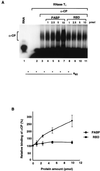
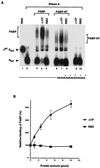
We previously identified a sequence-specific erythroid cell-enriched endoribonuclease (ErEN) activity involved in the turnover of the stable alpha-globin mRNA. We now demonstrate that ErEN activity is regulated by the poly(A) tail. The unadenylated alpha-globin 3' untranslated region (3'UTR) was an efficient substrate for ErEN cleavage, while the polyadenylated 3'UTR was inefficiently cleaved in an in vitro decay assay. The influence of the poly(A) tail was mediated through the poly(A)-binding protein (PABP) bound to the poly(A) tail, which can inhibit ErEN activity. ErEN cleavage of an adenylated alpha-globin 3'UTR was accentuated upon depletion of PABP from the cytosolic extract, while addition of recombinant PABP reestablished the inhibition of endoribonuclease cleavage. PABP inhibited ErEN activity indirectly through an interaction with the alphaCP mRNA stability protein. Sequestration of alphaCP resulted in an increase of ErEN cleavage activity, regardless of the polyadenylation state of the RNA. Using electrophoretic mobility shift assays, PABP was shown to enhance the binding efficiency of alphaCP to the alpha-globin 3'UTR, which in turn protected the ErEN target sequence. Conversely, the binding of PABP to the poly(A) tail was also augmented by alphaCP, implying that a stable higher-order structural network is involved in stabilization of the alpha-globin mRNA. Upon deadenylation, the interaction of PABP with alphaCP would be disrupted, rendering the alpha-globin 3'UTR more susceptible to endoribonuclease cleavage. The data demonstrated a specific role for PABP in protecting the body of an mRNA in addition to demonstrating PABP's well-characterized effect of stabilizing the poly(A) tail.
Figures


Similar articles
-
An erythroid-enriched endoribonuclease (ErEN) involved in alpha-globin mRNA turnover.Protein Pept Lett. 2007;14(2):131-6. doi: 10.2174/092986607779816168. Protein Pept Lett. 2007. PMID: 17305599 Review.
-
An mRNA stability complex functions with poly(A)-binding protein to stabilize mRNA in vitro.Mol Cell Biol. 1999 Jul;19(7):4552-60. doi: 10.1128/MCB.19.7.4552. Mol Cell Biol. 1999. PMID: 10373504 Free PMC article.
-
Assembly of the alpha-globin mRNA stability complex reflects binary interaction between the pyrimidine-rich 3' untranslated region determinant and poly(C) binding protein alphaCP.Mol Cell Biol. 1999 Jul;19(7):4572-81. doi: 10.1128/MCB.19.7.4572. Mol Cell Biol. 1999. PMID: 10373506 Free PMC article.
-
Characterization and purification of a mammalian endoribonuclease specific for the alpha -globin mRNA.J Biol Chem. 2002 Jan 25;277(4):2597-604. doi: 10.1074/jbc.M108330200. Epub 2001 Nov 15. J Biol Chem. 2002. PMID: 11711537
-
Regulation of alpha-globin mRNA stability.Exp Biol Med (Maywood). 2003 Apr;228(4):387-95. doi: 10.1177/153537020322800409. Exp Biol Med (Maywood). 2003. PMID: 12671183 Review.
Cited by
-
Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression.Genome Biol. 2003;4(7):223. doi: 10.1186/gb-2003-4-7-223. Epub 2003 Jul 1. Genome Biol. 2003. PMID: 12844354 Free PMC article. Review.
-
The role of deadenylation in the degradation of unstable mRNAs in trypanosomes.Nucleic Acids Res. 2009 Sep;37(16):5511-28. doi: 10.1093/nar/gkp571. Epub 2009 Jul 13. Nucleic Acids Res. 2009. PMID: 19596809 Free PMC article.
-
Polysome-bound endonuclease PMR1 is targeted to stress granules via stress-specific binding to TIA-1.Mol Cell Biol. 2006 Dec;26(23):8803-13. doi: 10.1128/MCB.00090-06. Epub 2006 Sep 18. Mol Cell Biol. 2006. PMID: 16982678 Free PMC article.
-
The role of mammalian poly(A)-binding proteins in co-ordinating mRNA turnover.Biochem Soc Trans. 2012 Aug;40(4):856-64. doi: 10.1042/BST20120100. Biochem Soc Trans. 2012. PMID: 22817748 Free PMC article. Review.
-
Structure and RNA binding of the third KH domain of poly(C)-binding protein 1.Nucleic Acids Res. 2005 Feb 24;33(4):1213-21. doi: 10.1093/nar/gki265. Print 2005. Nucleic Acids Res. 2005. PMID: 15731341 Free PMC article.
References
-
- Albrecht G, Krowczynska A, Brawerman G. Configuration of beta-globin messenger RNA in rabbit reticulocytes. Identification of sites exposed to endogenous and exogenous nucleases. J Mol Biol. 1984;178:881–896. - PubMed
-
- Beelman C A, Parker R. Degradation of mRNA in eukaryotes. Cell. 1995;81:179–183. - PubMed
-
- Binder R, Gordon D A, Hwang S P, Williams D L. Estrogen-induced destabilization and associated degradation intermediates of apolipoprotein II mRNA. Prog Clin Biol Res. 1990;322:227–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
