Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly
- PMID: 10938112
- PMCID: PMC86110
- DOI: 10.1128/MCB.20.17.6354-6363.2000
Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly
Abstract
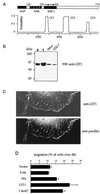
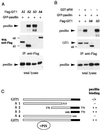
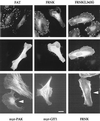
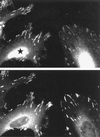
The p21-activated kinase PAK is targeted to focal complexes (FCs) through interactions with the SH3 domains of the PAK-interacting exchange factor PIX and Nck. PIX is a Rac GTP exchange factor that also binds the G-protein-coupled receptor kinase-interacting protein known as GIT1. Overexpression of GIT1 in fibroblasts or epithelial cells causes a loss of paxillin from FCs and stimulates cell motility. This is due to the direct interaction of a C-terminal 125-residue domain of GIT1 with paxillin, under the regulation of PIX. In its activated state, GIT1 can promote FC disassembly independent of actin-myosin contractile events. Additionally, GIT directly couples to a key component of FCs, focal adhesion kinase (FAK), via a conserved Spa2 homology domain. We propose that GIT1 and FAK cooperate to promote motility both by directly regulating focal complex dynamics and by the activation of Rac.
Figures






References
-
- Bagrodia S, Taylor S J, Jordon K A, Van Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. - PubMed
-
- Bagrodia S, Bailey D, Lenard Z, Hart M, Guan J L, Premont R T, Taylor S J, Cerione R A. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J Biol Chem. 1999;274:22393–22400. - PubMed
-
- Bagrodia S, Cerione R A. Pak to the future. Trends Cell Biol. 1999;9:350–355. - PubMed
-
- Brown M C, Curtis M S, Turner C E. Paxillin LD motifs may define a new family of protein recognition domains. Nat Struct Biol. 1998;5:677–678. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
