Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in Dhurrin biosynthesis
- PMID: 10938360
- PMCID: PMC59100
- DOI: 10.1104/pp.123.4.1437
Transgenic tobacco and Arabidopsis plants expressing the two multifunctional sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in Dhurrin biosynthesis
Abstract
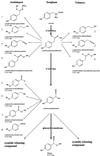
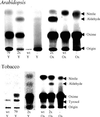
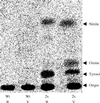
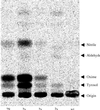
Novel cyanogenic plants have been generated by the simultaneous expression of the two multifunctional sorghum (Sorghum bicolor [L.] Moench) cytochrome P450 enzymes CYP79A1 and CYP71E1 in tobacco (Nicotiana tabacum cv Xanthi) and Arabidopsis under the regulation of the constitutive 35S promoter. CYP79A1 and CYP71E1 catalyze the conversion of the parent amino acid tyrosine to p-hydroxymandelonitrile, the aglycone of the cyanogenic glucoside dhurrin. CYP79A1 catalyzes the conversion of tyrosine to p-hydroxyphenylacetaldoxime and CYP71E1, the subsequent conversion to p-hydroxymandelonitrile. p-Hydroxymandelonitrile is labile and dissociates into p-hydroxybenzaldehyde and hydrogen cyanide, the same products released from dhurrin upon cell disruption as a result of pest or herbivore attack. In transgenic plants expressing CYP79A1 as well as CYP71E1, the activity of CYP79A1 is higher than that of CYP71E1, resulting in the accumulation of several p-hydroxyphenylacetaldoxime-derived products in the addition to those derived from p-hydroxymandelonitrile. Transgenic tobacco and Arabidopsis plants expressing only CYP79A1 accumulate the same p-hydroxyphenylacetaldoxime-derived products as transgenic plants expressing both sorghum cytochrome P450 enzymes. In addition, the transgenic CYP79A1 Arabidopsis plants accumulate large amounts of p-hydroxybenzylglucosinolate. In transgenic Arabidopsis expressing CYP71E1, this enzyme and the enzymes of the pre-existing glucosinolate pathway compete for the p-hydroxyphenylacetaldoxime as substrate, resulting in the formation of small amounts of p-hydroxybenzylglucosinolate. Cyanogenic glucosides are phytoanticipins, and the present study demonstrates the feasibility of expressing cyanogenic compounds in new plant species by gene transfer technology to improve pest and disease resistance.
Figures

References
-
- Bak S, Kahn RA, Nielsen HL, Møller BL, Halkier BA. Cloning of three A-type cytochromes P450, CYP71E1, CYP98 and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol. 1998;36:393–405. - PubMed
-
- Bak S, Olsen CE, Petersen BL, Møller BL, Halkier BA. Metabolic engineering of p-hydroxybenzyl-glucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J. 1999;20:663–672. - PubMed
-
- Conn EE. Cyanogenic glycosides. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants: A Comprehensive Treatise. 7: Secondary Plant Products. New York: Academic Press; 1981. pp. 479–500.
-
- Ehmann A. Identification of 2-O-(indole-3-acetyl)-d-glucopyranose, 4-O-(indole-3-acetyl)-d-glucopyranose and 6-O-(indole-3-acetyl)-d-glucopyranose from kernels of Zea mays by gas-liquid chromatography-mass spectrometry. Carbohydr Res. 1974;34:99–114. - PubMed
-
- Epstein J. Estimation of microquantities of cyanide. Anal Chem. 1947;19:272–274.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

