Spy1, a histidine-containing phosphotransfer signaling protein, regulates the fission yeast cell cycle through the Mcs4 response regulator
- PMID: 10940030
- PMCID: PMC111366
- DOI: 10.1128/JB.182.17.4868-4874.2000
Spy1, a histidine-containing phosphotransfer signaling protein, regulates the fission yeast cell cycle through the Mcs4 response regulator
Abstract
Common histidine-to-aspartate (His-to-Asp) phosphorelay signaling systems involve three types of signaling components: a sensor His kinase, a response regulator, and a histidine-containing phosphotransfer (HPt) protein. In the fission yeast Schizosaccharomyces pombe, two response regulators, Mcs4 and Prr1, have been identified recently, and it was shown that they are involved in the signal transduction implicated in stress responses. Furthermore, Mcs4 appears to be involved in mitotic cell-cycle control. However, neither the HPt phosphotransmitter nor His kinase has been characterized in S. pombe. In this study, we identified a gene encoding an HPt phosphotransmitter, named Spy1 (S. pombe YPD1-like protein). The spy1(+) gene showed an ability to complement a mutational lesion of the Saccharomyces cerevisiae YPD1 gene, which is involved in an osmosensing signal transduction. The result from yeast two-hybrid analysis indicated that Spy1 interacts with Mcs4. To gain insight into the function of Spy1, a series of genetic analyses were conducted. The results provided evidence that Spy1, together with Mcs4, plays a role in regulation of the G(2)/M cell cycle progression. Spy1-deficient cells appear to be precocious in the entry to M phase. In the proposed model, Spy1 modulates Mcs4 in a negative manner, presumably through a direct His-to-Asp phosphorelay, operating upstream of the Sty1 mitogen-activated protein kinase cascade.
Figures



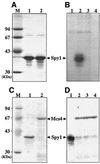


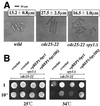
References
-
- Aiba H, Mizuno T, Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of ompF and ompC genes in Escherichia coli. J Biol Chem. 1989;264:8563–8567. - PubMed
-
- Aiba H, Yamada H, Ohmiya R, Mizuno T. The osmo-inducible gpd1+ gene is a target of the signaling pathway involving Wis1 MAP-kinase kinase in fission yeast. FEBS Lett. 1995;376:199–201. - PubMed
-
- Appleby J L, Parkinson J S, Bourret R B. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. - PubMed
-
- Bourret R B, Borvich K A, Simon M I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

