Cell-associated pheromone peptide (cCF10) production and pheromone inhibition in Enterococcus faecalis
- PMID: 10940037
- PMCID: PMC111373
- DOI: 10.1128/JB.182.17.4926-4933.2000
Cell-associated pheromone peptide (cCF10) production and pheromone inhibition in Enterococcus faecalis
Abstract
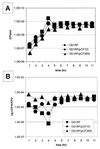
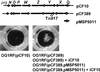
In Enterococcus faecalis, the peptide cCF10 acts as a pheromone, inducing transfer of the conjugative plasmid pCF10 from plasmid-containing donor cells to plasmid-free recipient cells. In these studies, it was found that a substantial amount of cCF10 associates with the envelope of the producing cell. Pheromone activity was detected in both wall and membrane fractions, with the highest activity associated with the wall. Experiments examining the effects of protease inhibitor treatments either prior to or following cell fractionation suggested the presence of a cell envelope-associated pro-cCF10 that can be processed to mature cCF10 by a maturase or protease. A pCF10-encoded membrane protein, PrgY, was shown to prevent self-induction of donor cells by reducing the level of pheromone activity in the cell wall fraction.
Figures





Similar articles
-
The peptide pheromone-inducible conjugation system of Enterococcus faecalis plasmid pCF10: cell-cell signalling, gene transfer, complexity and evolution.Philos Trans R Soc Lond B Biol Sci. 2007 Jul 29;362(1483):1185-93. doi: 10.1098/rstb.2007.2043. Philos Trans R Soc Lond B Biol Sci. 2007. PMID: 17360276 Free PMC article. Review.
-
ccfA, the genetic determinant for the cCF10 peptide pheromone in Enterococcus faecalis OG1RF.J Bacteriol. 2002 Feb;184(4):1155-62. doi: 10.1128/jb.184.4.1155-1162.2002. J Bacteriol. 2002. PMID: 11807076 Free PMC article.
-
Specific control of endogenous cCF10 pheromone by a conserved domain of the pCF10-encoded regulatory protein PrgY in Enterococcus faecalis.J Bacteriol. 2005 Jul;187(14):4830-43. doi: 10.1128/JB.187.14.4830-4843.2005. J Bacteriol. 2005. PMID: 15995198 Free PMC article.
-
Characterization of the sequence specificity determinants required for processing and control of sex pheromone by the intramembrane protease Eep and the plasmid-encoded protein PrgY.J Bacteriol. 2008 Feb;190(4):1172-83. doi: 10.1128/JB.01327-07. Epub 2007 Dec 14. J Bacteriol. 2008. PMID: 18083822 Free PMC article.
-
Peptide pheromone-induced transfer of plasmid pCF10 in Enterococcus faecalis: probing the genetic and molecular basis for specificity of the pheromone response.Peptides. 2001 Oct;22(10):1529-39. doi: 10.1016/s0196-9781(01)00489-2. Peptides. 2001. PMID: 11587782 Review.
Cited by
-
Molecular Mechanisms of Virulence Regulation in Staphylococcus aureus: A Journey into Reconstitutive Biochemistry.Acc Chem Res. 2025 May 20;58(10):1657-1669. doi: 10.1021/acs.accounts.5c00117. Epub 2025 May 7. Acc Chem Res. 2025. PMID: 40331756
-
The peptide pheromone-inducible conjugation system of Enterococcus faecalis plasmid pCF10: cell-cell signalling, gene transfer, complexity and evolution.Philos Trans R Soc Lond B Biol Sci. 2007 Jul 29;362(1483):1185-93. doi: 10.1098/rstb.2007.2043. Philos Trans R Soc Lond B Biol Sci. 2007. PMID: 17360276 Free PMC article. Review.
-
Analysis of the amino acid sequence specificity determinants of the enterococcal cCF10 sex pheromone in interactions with the pheromone-sensing machinery.J Bacteriol. 2007 Feb;189(4):1399-406. doi: 10.1128/JB.01226-06. Epub 2006 Nov 10. J Bacteriol. 2007. PMID: 17098891 Free PMC article.
-
ccfA, the genetic determinant for the cCF10 peptide pheromone in Enterococcus faecalis OG1RF.J Bacteriol. 2002 Feb;184(4):1155-62. doi: 10.1128/jb.184.4.1155-1162.2002. J Bacteriol. 2002. PMID: 11807076 Free PMC article.
-
Role of the Enterococcus faecalis GelE protease in determination of cellular chain length, supernatant pheromone levels, and degradation of fibrin and misfolded surface proteins.J Bacteriol. 2003 Jun;185(12):3613-23. doi: 10.1128/JB.185.12.3613-3623.2003. J Bacteriol. 2003. PMID: 12775699 Free PMC article.
References
-
- An F Y, Clewell D B. Characterization of the determinant (traB) encoding sex pheromone shutdown by the hemolysin/bacteriocin plasmid pAD1 in Enterococcus faecalis. Plasmid. 1994;31:215–221. - PubMed
-
- Bae T, Clerc-Bardin S, Dunny G M. Analysis of expression of prgX, a key negative regulator of the transfer of the Enterococcus faecalis pheromone-inducible plasmid pCF10. J Mol Biol. 2000;297:861–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

