Effects of limited aeration and of the ArcAB system on intermediary pyruvate catabolism in Escherichia coli
- PMID: 10940038
- PMCID: PMC111374
- DOI: 10.1128/JB.182.17.4934-4940.2000
Effects of limited aeration and of the ArcAB system on intermediary pyruvate catabolism in Escherichia coli
Abstract
The capacity of Escherichia coli to adapt its catabolism to prevailing redox conditions resides mainly in three catabolic branch points involving (i) pyruvate formate-lyase (PFL) and the pyruvate dehydrogenase complex (PDHc), (ii) the exclusively fermentative enzymes and those of the Krebs cycle, and (iii) the alternative terminal cytochrome bd and cytochrome bo oxidases. A quantitative analysis of the relative catabolic fluxes through these pathways is presented for steady-state glucose-limited chemostat cultures with controlled oxygen availability ranging from full aerobiosis to complete anaerobiosis. Remarkably, PFL contributed significantly to the catabolic flux under microaerobic conditions and was found to be active simultaneously with PDHc and cytochrome bd oxidase-dependent respiration. The synthesis of PFL and cytochrome bd oxidase was found to be maximal in the lower microaerobic range but not in a delta ArcA mutant, and we conclude that the Arc system is more active with respect to regulation of these two positively regulated operons during microaerobiosis than during anaerobiosis.
Figures
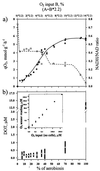
 , chemostat B) and intracellular redox state (NADH/NAD ratio) (□, chemostat A; ⊡, chemostat B) in response to a change in the oxygen supply in wild-type, glucose-limited E. coli. Upper x axis, actual percentage of oxygen in the inflowing gas in chemostat B. It took 2.2-fold more oxygen in the inflowing gas to evoke the same effect in chemostat A. (b) Residual dissolved oxygen concentrations (○) in steady-state glucose-limited chemostat cultures of E. coli (wild type) with increasing oxygen supply. Bars, amplitude of fluctuations in DOT. (Inset) Control of O2 electrode sensitivity in the low (below 1 μM O2) and high ranges. The control was performed in the absence of cells.
, chemostat B) and intracellular redox state (NADH/NAD ratio) (□, chemostat A; ⊡, chemostat B) in response to a change in the oxygen supply in wild-type, glucose-limited E. coli. Upper x axis, actual percentage of oxygen in the inflowing gas in chemostat B. It took 2.2-fold more oxygen in the inflowing gas to evoke the same effect in chemostat A. (b) Residual dissolved oxygen concentrations (○) in steady-state glucose-limited chemostat cultures of E. coli (wild type) with increasing oxygen supply. Bars, amplitude of fluctuations in DOT. (Inset) Control of O2 electrode sensitivity in the low (below 1 μM O2) and high ranges. The control was performed in the absence of cells.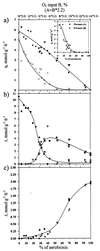
 , chemostat B) formation in response to change in oxygen availability in wild-type E. coli. For an explanation of x-axis values, see the Fig. 2 legend. (b) Effect of oxygen availability on distribution of in vivo flux (J) between PFL (■, chemostat A; ◘, chemostat B) and PDHc (◊, chemostat A; ·⃟, chemostat B) in wild-type E. coli. (c) Effect of oxygen availability on in vivo TCA activity (
, chemostat B) formation in response to change in oxygen availability in wild-type E. coli. For an explanation of x-axis values, see the Fig. 2 legend. (b) Effect of oxygen availability on distribution of in vivo flux (J) between PFL (■, chemostat A; ◘, chemostat B) and PDHc (◊, chemostat A; ·⃟, chemostat B) in wild-type E. coli. (c) Effect of oxygen availability on in vivo TCA activity ( , chemostat A;
, chemostat A;  , chemostat B) in wild-type E. coli.
, chemostat B) in wild-type E. coli.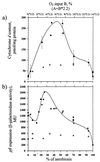
 , chemostat B) in wild-type and ΔarcA (Δ) strains of E. coli.
, chemostat B) in wild-type and ΔarcA (Δ) strains of E. coli.References
-
- Becker S, Vlad D, Schuster S, Pfeiffer P, Unden G. Regulatory O2 tensions for the synthesis of fermentation products in Escherichia coli and relation to aerobic respiration. Arch Microbiol. 1997;168:290–296. - PubMed
-
- Bogachev A, Murtazina R, Shestopalov A, Skulachev V. Induction of the Escherichia coli cytochrome d by low ΔμH+ and by sodium ions. Eur J Biochem. 1995;232:304–308. - PubMed
-
- Cassey B, Guest J R, Attwood M M. Environmental control of pyruvate dehydrogenase complex expression in Escherichia coli. FEMS Microbiol Lett. 1998;159:325–329. - PubMed
-
- Clark D P. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989;5:223–234. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

