Inhibition of nitric oxide synthesis and gene knockout of neuronal nitric oxide synthase impaired adaptation of mouse optokinetic response eye movements
- PMID: 10940322
- PMCID: PMC311332
- DOI: 10.1101/lm.7.4.220
Inhibition of nitric oxide synthesis and gene knockout of neuronal nitric oxide synthase impaired adaptation of mouse optokinetic response eye movements
Abstract
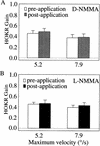
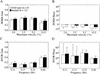
Nitric oxide (NO) plays a key role in synaptic transmission efficiency in the central nervous system. To gain an insight on the role of NO in cerebellar functions, we, here, measured the dynamics of the horizontal optokinetic response (HOKR) and vestibulo-ocular reflex (HVOR), and the adaptation of HOKR in mice locally injected with N(G)-monomethyl-L-arginine (L-NMMA) that inhibits NO synthesis and in mice devoid of neuronal nitric oxide synthase (nNOS). Local application of L-NMMA into the cerebellar flocculi induced no change in the dynamics of the HOKR but markedly depressed the adaptation of the HOKR induced by 1 hr of sustained screen oscillation. A slight difference was seen in the HOKR but not in the HVOR dynamics between nNOS(-/-) mutant and wild-type mice. One hour of sustained screen oscillation induced adaptation of the HOKR gains in wild-type mice but not in mutants. These observations suggest that NO is essential for the adaptation of the HOKR and that nNOS is the major enzyme for NO synthesis in the process.
Figures


Similar articles
-
Loss of adaptability of horizontal optokinetic response eye movements in mGluR1 knockout mice.Neurosci Res. 2002 Feb;42(2):141-5. doi: 10.1016/s0168-0102(01)00308-x. Neurosci Res. 2002. PMID: 11849733
-
Dynamic characteristics and adaptability of mouse vestibulo-ocular and optokinetic response eye movements and the role of the flocculo-olivary system revealed by chemical lesions.Proc Natl Acad Sci U S A. 1998 Jun 23;95(13):7705-10. doi: 10.1073/pnas.95.13.7705. Proc Natl Acad Sci U S A. 1998. PMID: 9636214 Free PMC article.
-
Role of protein kinase C family in the cerebellum-dependent adaptive learning of horizontal optokinetic response eye movements in mice.Eur J Neurosci. 2003 Jul;18(1):134-42. doi: 10.1046/j.1460-9568.2003.02717.x. Eur J Neurosci. 2003. PMID: 12859346
-
Selective inhibitors of neuronal nitric oxide synthase--is no NOS really good NOS for the nervous system?Trends Pharmacol Sci. 1997 Jun;18(6):204-11. doi: 10.1016/s0165-6147(97)01064-x. Trends Pharmacol Sci. 1997. PMID: 9226999 Review.
-
Neuronal and endothelial nitric oxide synthase gene knockout mice.Braz J Med Biol Res. 1999 Nov;32(11):1353-9. doi: 10.1590/s0100-879x1999001100005. Braz J Med Biol Res. 1999. PMID: 10559836 Review.
Cited by
-
Interneuronal NMDA receptors regulate long-term depression and motor learning in the cerebellum.J Physiol. 2019 Feb;597(3):903-920. doi: 10.1113/JP276794. Epub 2018 Nov 24. J Physiol. 2019. PMID: 30382582 Free PMC article.
-
Enhancement of both long-term depression induction and optokinetic response adaptation in mice lacking delphilin.PLoS One. 2008 May 28;3(5):e2297. doi: 10.1371/journal.pone.0002297. PLoS One. 2008. PMID: 18509461 Free PMC article.
-
Role of cerebellar cortical protein synthesis in transfer of memory trace of cerebellum-dependent motor learning.J Neurosci. 2011 Jun 15;31(24):8958-66. doi: 10.1523/JNEUROSCI.1151-11.2011. J Neurosci. 2011. PMID: 21677179 Free PMC article.
-
Nitric Oxide Participates in the Brain Ischemic Tolerance Induced by Intermittent Hypobaric Hypoxia in the Hippocampal CA1 Subfield in Rats.Neurochem Res. 2018 Sep;43(9):1779-1790. doi: 10.1007/s11064-018-2593-9. Epub 2018 Jul 11. Neurochem Res. 2018. PMID: 29995175
-
Type 1 inositol trisphosphate receptor regulates cerebellar circuits by maintaining the spine morphology of purkinje cells in adult mice.J Neurosci. 2013 Jul 24;33(30):12186-96. doi: 10.1523/JNEUROSCI.0545-13.2013. J Neurosci. 2013. PMID: 23884927 Free PMC article.
References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Boxall AR, Garthwaite J. Long-term depression in rat cerebellum requires both NO synthase and NO-sensitive guanylyl cyclase. Eur J Neurosci. 1996;8:2209–2212. - PubMed
-
- Chapman PF, Atkins CM, Allen MT, Haley JE, Steinmetz JE. Inhibition of nitric oxide synthesis impairs two different forms of learning. NeuroReport. 1992;3:567–570. - PubMed
-
- Crepel F, Jaillard D. Protein kinases, nitric oxide and long-term depression of synapses in the cerebellum. NeuroReport. 1990;1:133–136. - PubMed
-
- Crepel F, Hemart D, Jaillard D, Daniel H. Cellular mechanisms of long-term depression in the cerebellum. Behav Brain Sci. 1996;19:347–353.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
