Crystal structure of the matrix protein VP40 from Ebola virus
- PMID: 10944105
- PMCID: PMC302032
- DOI: 10.1093/emboj/19.16.4228
Crystal structure of the matrix protein VP40 from Ebola virus
Abstract
Ebola virus maturation occurs at the plasma membrane of infected cells and involves the clustering of the viral matrix protein VP40 at the assembly site as well as its interaction with the lipid bilayer. Here we report the X-ray crystal structure of VP40 from Ebola virus at 2.0 A resolution. The crystal structure reveals that Ebola virus VP40 is topologically distinct from all other known viral matrix proteins, consisting of two domains with unique folds, connected by a flexible linker. The C-terminal domain, which is absolutely required for membrane binding, contains large hydrophobic patches that may be involved in the interaction with lipid bilayers. Likewise, a highly basic region is shared between the two domains. The crystal structure reveals how the molecule may be able to switch from a monomeric conformation to a hexameric form, as observed in vitro. Its implications for the assembly process are discussed.
Figures

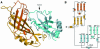

References
-
- Becker S., Spiess,M. and Klenk,H.D. (1995) The asialoglycoprotein receptor is a potential liver-specific receptor for Marburg virus. J. Gen. Virol., 76, 393–399. - PubMed
-
- Brünger A.T. et al. (1998) Crystallographic and NMR system (CNS): a new software system for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
-
- Bukreyev A.A., Volchkov,V.E., Blinov,V.M. and Netesov,S.V. (1993) The Vp35 and VP40 proteins of filoviruses. Homology between Marburg and Ebola viruses. FEBS Lett., 322, 41–46. - PubMed
-
- Christensen A.M., Massiah,M.A., Turner,B.G., Sundquist,W.I. and Summers,M.F. (1996) Three-dimensional structure of the HTLV-II matrix protein and comparative analysis of matrix proteins from different classes of pathogenic human retroviruses. J. Mol. Biol., 264, 1117–1131. - PubMed
-
- Collaborative Computational Project Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–776. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

