Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90
- PMID: 10944114
- PMCID: PMC302037
- DOI: 10.1093/emboj/19.16.4310
Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90
Abstract
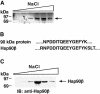
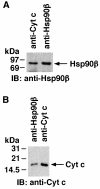
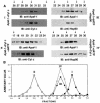
The release of cytochrome c from mitochondria results in the formation of an Apaf-1-caspase-9 apoptosome and induces the apoptotic protease cascade by activation of procaspase-3. The present studies demonstrate that heat shock protein 90 (Hsp90) forms a cytosolic complex with Apaf-1 and thereby inhibits the formation of the active complex. Immunodepletion of Hsp90 depletes Apaf-1 and thereby inhibits cytochrome c-mediated activation of caspase-9. Addition of purified Apaf-1 to Hsp90-depleted cytosolic extracts restores cytochrome c-mediated activation of procaspase-9. We also show that Hsp90 inhibits cytochrome c-mediated oligomerization of Apaf-1 and thereby activation of procaspase-9. Furthermore, treatment of cells with diverse DNA-damaging agents dissociates the Hsp90-Apaf-1 complex and relieves the inhibition of procaspase-9 activation. These findings provide the first evidence for a negative cytosolic regulator of cytochrome c-dependent apoptosis and for involvement of a chaperone in the caspase cascade.
Figures

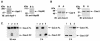






Similar articles
-
Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3.Oncogene. 2000 Apr 13;19(16):1975-81. doi: 10.1038/sj.onc.1203531. Oncogene. 2000. PMID: 10803458
-
Negative regulation of the Apaf-1 apoptosome by Hsp70.Nat Cell Biol. 2000 Aug;2(8):476-83. doi: 10.1038/35019510. Nat Cell Biol. 2000. PMID: 10934467
-
Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome.Nat Cell Biol. 2000 Aug;2(8):469-75. doi: 10.1038/35019501. Nat Cell Biol. 2000. PMID: 10934466
-
Apaf-1: Regulation and function in cell death.Biochimie. 2017 Apr;135:111-125. doi: 10.1016/j.biochi.2017.02.001. Epub 2017 Feb 9. Biochimie. 2017. PMID: 28192157 Review.
-
Chemical-induced apoptosis: formation of the Apaf-1 apoptosome.Drug Metab Rev. 2003 Nov;35(4):337-63. doi: 10.1081/dmr-120026497. Drug Metab Rev. 2003. PMID: 14705865 Review.
Cited by
-
The heat shock proteins as targets for radiosensitization and chemosensitization in cancer.Cancer Biol Ther. 2011 Dec 15;12(12):1023-31. doi: 10.4161/cbt.12.12.18374. Epub 2011 Dec 15. Cancer Biol Ther. 2011. PMID: 22236878 Free PMC article. Review.
-
Quantitative proteomics of heat-treated human cells show an across-the-board mild depletion of housekeeping proteins to massively accumulate few HSPs.Cell Stress Chaperones. 2015 Jul;20(4):605-20. doi: 10.1007/s12192-015-0583-2. Epub 2015 Apr 8. Cell Stress Chaperones. 2015. PMID: 25847399 Free PMC article.
-
The Apaf-1-binding protein Aven is cleaved by Cathepsin D to unleash its anti-apoptotic potential.Cell Death Differ. 2012 Sep;19(9):1435-45. doi: 10.1038/cdd.2012.17. Epub 2012 Mar 2. Cell Death Differ. 2012. PMID: 22388353 Free PMC article.
-
Cytotoxicity of bismuth(III) dithiocarbamate derivatives by promoting a mitochondrial-dependent apoptotic pathway and suppressing MCF-7 breast adenocarcinoma cell invasion.J Biol Inorg Chem. 2024 Mar;29(2):217-241. doi: 10.1007/s00775-023-02041-x. Epub 2024 Feb 18. J Biol Inorg Chem. 2024. PMID: 38369679
-
Geldanamycin induces production of heat shock protein 70 and partially attenuates ototoxicity caused by gentamicin in the organ of Corti explants.J Biomed Sci. 2009 Sep 2;16(1):79. doi: 10.1186/1423-0127-16-79. J Biomed Sci. 2009. PMID: 19723345 Free PMC article.
References
-
- Adrain C., Slee,E., Harte,M. and Martin,S. (1999) Regulation of apoptotic protease activating factor-1 oligomerization and apoptosis by the WD-40 repeat region. J. Biol. Chem., 274, 20855–20860. - PubMed
-
- Alnemri E.S., Livingston,D.J., Nicholson,D.W., Salvesen,G., Thornberry,N.A., Wong,W.W. and Yuan,J. (1996) Human ICE/CED-3 protease nomenclature. Cell, 87, 171. - PubMed
-
- Benedict M., Hu,Y., Inohara,N. and Nunez,G. (2000) Expression and functional analysis of Apaf-1 isoforms. Extra WD-40 repeat is required for cytochrome c binding and regulated activation of procaspase-9. J. Biol. Chem., 275, 8461–8468. - PubMed
-
- Bertram J., Palfner,K., Hiddemann,W. and Kneba,M. (1996) Increase of P-glycoprotein-mediated drug resistance by hsp90 β. Anti-Cancer Drugs, 7, 838–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

