Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3
- PMID: 10944117
- PMCID: PMC302030
- DOI: 10.1093/emboj/19.16.4342
Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3
Abstract
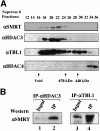
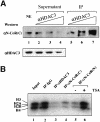
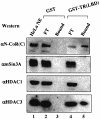
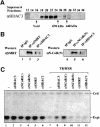
We present evidence that both corepressors SMRT and N-CoR exist in large protein complexes with estimated sizes of 1.5-2 MDa in HeLa nuclear extracts. Using a combination of conventional and immunoaffinity chromatography, we have successfully isolated a SMRT complex and identified histone deacetylase 3 (HDAC3) and transducin (beta)-like I (TBL1), a WD-40 repeat-containing protein, as the subunits of the purified SMRT complex. We show that the HDAC3-containing SMRT and N-CoR complexes can bind to unliganded thyroid hormone receptors (TRs) in vitro. We demonstrate further that in Xenopus oocytes, both SMRT and N-CoR also associate with HDAC3 in large protein complexes and that injection of antibodies against HDAC3 or SMRT/N-CoR led to a partial relief of repression by unliganded TR/RXR. These findings thus establish both SMRT and N-CoR complexes as bona fide HDAC-containing complexes and shed new light on the molecular pathways by which N-CoR and SMRT function in transcriptional repression.
Figures




References
-
- Alland L., Muhle,R., Hou,H.,Jr, Potes,J., Chin,L., Schreiber-Agus,N. and DePinho,R.A. (1997) Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature, 387, 49–55. - PubMed
-
- Baniahmad A., Steiner,C., Kohne,A.C. and Renkawitz,R. (1990) Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell, 61, 505–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

