Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure
- PMID: 10944177
- PMCID: PMC2270055
- DOI: 10.1111/j.1469-7793.2000.t01-1-00139.x
Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure
Abstract
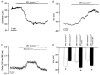
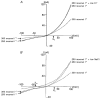
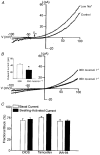
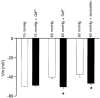
1. Increases in intravascular pressure depolarize vascular smooth muscle cells. Based on the attenuating effects of Cl- channel antagonists, it has been suggested that swelling-activated Cl- channels may be integral to this response. Consequently, this study tested for the presence of a swelling-activated Cl- conductance in both intact rat cerebral arteries and isolated rat smooth muscle cells. 2. A 50 mosmol l-1 hyposmotic challenge (300 to 250 mosmol l-1) constricted rat cerebral arteries. This constriction contained all the salient features of a pressure-induced response including smooth muscle cell depolarization and a rise in intracellular Ca2+ that was blocked by voltage-operated Ca2+ channel antagonists. The hyposmotically induced depolarization was attenuated by DIDS (300 microM) and tamoxifen (1 microM), a response consistent with the presence of a swelling-activated Cl- conductance. 3. A swelling-activated current was identified in cerebral vascular smooth muscle cells. This current was sensitive to Cl- channel antagonists including DIDS (300 microM), tamoxifen (1 microM) and IAA-94 (100 microM). However, contrary to expectations, the reversal potential of this swelling-activated current shifted with the Na+ equilibrium potential and not the Cl- equilibrium potential, indicating that the swelling-activated current was carried by cations and not anions. The swelling-activated cation current was blocked by Gd3+, a cation channel antagonist. 4. Gd3+ also blocked both swelling- and pressure-induced depolarization of smooth muscle cells in intact cerebral arteries. 5. These findings suggest that swelling- and pressure-induced depolarization arise from the activation of a cation conductance. This current is inhibited by DIDS, tamoxifen, IAA-94 and gadolinium.
Figures



References
-
- Allen MC, Newland C, Valverde MA, Hardy SP. Inhibition of ligand-gated cation-selective channels by tamoxifen. European Journal of Pharmacology. 1998;354:261–269. - PubMed
-
- Brayden JE, Wellman GC. Endothelium-dependent dilation of feline cerebral arteries: role of membrane potential and cyclic nucleotides. Journal of Cerebral Blood Flow and Metabolism. 1989;9:256–263. - PubMed
-
- Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. American Journal of Physiology. 1992;262:C1083–1088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

