TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein
- PMID: 10944200
- PMCID: PMC27728
- DOI: 10.1073/pnas.170295097
TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein
Abstract
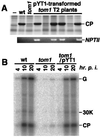
Host-encoded factors play an important role in virus multiplication, acting in concert with virus-encoded factors. However, information regarding the host factors involved in this process is limited. Here we report the map-based cloning of an Arabidopsis thaliana gene, TOM1, which is necessary for the efficient multiplication of tobamoviruses, positive-strand RNA viruses infecting a wide variety of plants. The TOM1 mRNA is suggested to encode a 291-aa polypeptide that is predicted to be a multipass transmembrane protein. The Sos recruitment assay supported the hypothesis that TOM1 is associated with membranes, and in addition, that TOM1 interacts with the helicase domain of tobamovirus-encoded replication proteins. Taken into account that the tobamovirus replication complex is associated with membranes, we propose that TOM1 participates in the in vivo formation of the replication complex by serving as a membrane anchor.
Figures



References
-
- Lai M M C. Virology. 1998;244:1–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

