Intramolecular control of transcriptional activity by the NK2-specific domain in NK-2 homeodomain proteins
- PMID: 10944215
- PMCID: PMC16883
- DOI: 10.1073/pnas.97.17.9443
Intramolecular control of transcriptional activity by the NK2-specific domain in NK-2 homeodomain proteins
Abstract
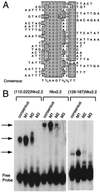
The developmentally important homeodomain transcription factors of the NK-2 class contain a highly conserved region, the NK2-specific domain (NK2-SD). The function of this domain, however, remains unknown. The primary structure of the NK2-SD suggests that it might function as an accessory DNA-binding domain or as a protein-protein interaction interface. To assess the possibility that the NK2-SD may contribute to DNA-binding specificity, we used a PCR-based approach to identify a consensus DNA-binding sequences for Nkx2.2, an NK-2 family member involved in pancreas and central nervous system development. The consensus sequence (T(C)(T)AAGT(G)(A)(G)(C)TT) is similar to the known binding sequences for other NK-2 homeodomain proteins, but we show that the NK2-SD does not contribute significantly to specific DNA binding to this sequence. To determine whether the NK2-SD contributes to transactivation, we used GAL4-Nkx2. 2 fusion constructs to map a powerful transcriptional activation domain in the C-terminal region beyond the conserved NK2-SD. Interestingly, this C-terminal region functions as a transcriptional activator only in the absence of an intact NK2-SD. The NK2-SD also can mask transactivation from the paired homeodomain transcription factor Pax6, but it has no effect on transcription by itself. These results demonstrate that the NK2-SD functions as an intramolecular regulator of the C-terminal activation domain in Nkx2.2 and support a model in which interactions through the NK2-SD regulate the ability of NK-2-class proteins to activate specific genes during development.
Figures




References
-
- Harvey R P. Dev Biol. 1996;178:203–216. - PubMed
-
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox C H, Ward J M, Gonzalez F J. Genes Dev. 1996;10:60–69. - PubMed
-
- Sussel L, Marin O, Kimura S, Rubenstein J L. Development (Cambridge, UK) 1999;126:3359–3370. - PubMed
-
- Lints T J, Parsons L M, Hartley L, Lyons I, Harvey R P. Development (Cambridge, UK) 1993;119:419–431. - PubMed
-
- Biben C, Harvey R P. Genes Dev. 1997;11:1357–1369. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

