Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype
- PMID: 10944224
- PMCID: PMC16907
- DOI: 10.1073/pnas.97.17.9579
Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype
Abstract
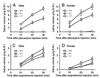
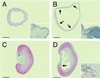
Muscarinic acetylcholine receptors consist of five distinct subtypes and have been important targets for drug development. In the periphery, muscarinic acetylcholine receptors mediate cholinergic signals to autonomic organs, but specific physiological functions of each subtype remain poorly elucidated. Here, we have constructed and analyzed mutant mice lacking the M(3) receptor and have demonstrated that this subtype plays key roles in salivary secretion, pupillary constriction, and bladder detrusor contractions. However, M(3)-mediated signals in digestive and reproductive organs are dispensable, likely because of redundant mechanisms through other muscarinic acetylcholine receptor subtypes or other mediators. In addition, we have found prominent urinary retention only in the male, which indicates a considerable sex difference in the micturition mechanism. Accordingly, this mutant mouse should provide a useful animal model for investigation of human diseases that are affected in the peripheral cholinergic functions.
Figures




References
-
- Mitchelson F. Pharmacol Ther. 1988;37:357–423. - PubMed
-
- Caulfield M P, Birdsall N J M. Pharmacol Rev. 1998;50:279–290. - PubMed
-
- Matsui M, Araki Y, Karasawa H, Matsubara N, Taketo M M, Seldin M F. Genes Genet Syst. 1999;74:15–21. - PubMed
-
- Ehlert F J, Sawyer G W, Esqueda E E. Life Sci. 1999;64:387–394. - PubMed
-
- Eglen R M, Choppin A, Dillon M P, Hegde S. Curr Opin Chem Biol. 1999;3:426–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

