Restoration of hemoglobin A synthesis in erythroid cells from peripheral blood of thalassemic patients
- PMID: 10944225
- PMCID: PMC16909
- DOI: 10.1073/pnas.97.17.9591
Restoration of hemoglobin A synthesis in erythroid cells from peripheral blood of thalassemic patients
Abstract
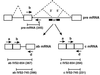
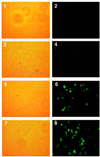
Mononuclear cells from peripheral blood of thalassemic patients were treated with morpholino oligonucleotides antisense to aberrant splice sites in mutant beta-globin precursor mRNAs (pre-mRNAs). The oligonucleotides restored correct splicing and translation of beta-globin mRNA, increasing the hemoglobin (Hb) A synthesis in erythroid cells from patients with IVS2-654/beta(E), IVS2-745/IVS2-745, and IVS2-745/IVS2-1 genotypes. The maximal Hb A level for repaired IVS2-745 mutation was approximately 30% of normal; Hb A was still detectable 9 days after a single treatment with oligonucleotide. Thus, expression of defective beta-globin genes was repaired and significant level of Hb A was restored in a cell population that would be targeted in clinical applications of this approach.
Figures
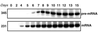





References
-
- Cohen A, editor. Cooley's Anemia: Progress in Biology and Medicine. Bethesda, MD: National Institutes of Health, National Heart, Blood and Lung Institute; 1995.
-
- Schwartz E, Benz E J, Jr, Forget B G. In: Hemalotogy, Basic Principles and Practice. Hoffman R, Benz E J, Shattil S J, Furie B, Cohen H J, editors. New York: Churchill Livingstone; 1995. pp. 586–610.
-
- Olivieri N F, Weatherall D J. Hum Mol Genet. 1998;7:1655–1658. - PubMed
-
- Olivieri N F. N Engl J Med. 1999;341:99–109. - PubMed
-
- Fucharoen S, Winichagoon P. Curr Opin Hematol. 2000;7:106–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

