Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst
- PMID: 10948103
- PMCID: PMC101694
- DOI: 10.1128/IAI.68.9.4900-4906.2000
Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst
Abstract
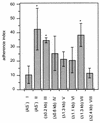
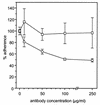
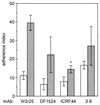
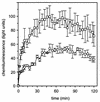
The aggregation substance (AS) of Enterococcus faecalis, encoded on sex pheromone plasmids, is a surface-bound glycoprotein that mediates aggregation between bacteria thereby facilitating plasmid transfer. Sequencing of the pAD1-encoded Asa1 revealed that this surface protein contains two RGD motifs which are known to ligate integrins. Therefore, we investigated the influence of AS on the interaction of E. faecalis with human monocyte-derived macrophages which constitutively express beta(2) integrins (e.g., CD18). AS was found to cause a greater-than-fivefold increase in enterococcal adherence to macrophages and a greater-than-sevenfold increase in phagocytosis. Adherence was mediated by an interaction between the RGD motif and the integrin CD11b/CD18 (complement receptor type 3) as demonstrated by inhibition studies with monoclonal antibodies and RGD peptide. AS-bearing enterococci were significantly more resistant to macrophage killing during the first 3 h postinfection, probably due to inhibition of the respiratory burst as indicated by reduced concentrations of superoxide anion.
Figures



Similar articles
-
Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism.FEMS Immunol Med Microbiol. 1999 Oct;26(1):49-60. doi: 10.1111/j.1574-695X.1999.tb01371.x. FEMS Immunol Med Microbiol. 1999. PMID: 10518042
-
Transcriptional control of sex-pheromone-inducible genes on plasmid pAD1 of Enterococcus faecalis and sequence analysis of a third structural gene for (pPD1-encoded) aggregation substance.Mol Microbiol. 1992 May;6(10):1297-308. doi: 10.1111/j.1365-2958.1992.tb00851.x. Mol Microbiol. 1992. PMID: 1640831
-
Sex pheromone plasmid pAD1-encoded aggregation substance of Enterococcus faecalis is positively regulated in trans by traE1.Eur J Biochem. 1993 May 15;214(1):333-8. doi: 10.1111/j.1432-1033.1993.tb17928.x. Eur J Biochem. 1993. PMID: 8508803
-
The sex pheromone system of Enterococcus faecalis. More than just a plasmid-collection mechanism?Eur J Biochem. 1994 Jun 1;222(2):235-46. doi: 10.1111/j.1432-1033.1994.tb18862.x. Eur J Biochem. 1994. PMID: 8020463 Review.
-
Sex pheromones and plasmid transfer in Enterococcus faecalis.Plasmid. 1989 May;21(3):175-84. doi: 10.1016/0147-619x(89)90041-3. Plasmid. 1989. PMID: 2550976 Review.
Cited by
-
Aggregation substance increases adherence and internalization, but not translocation, of Enterococcus faecalis through different intestinal epithelial cells in vitro.Infect Immun. 2000 Oct;68(10):6044-7. doi: 10.1128/IAI.68.10.6044-6047.2000. Infect Immun. 2000. PMID: 10992519 Free PMC article.
-
The aggregation domain of aggregation substance, not the RGD motifs, is critical for efficient internalization by HT-29 enterocytes.Infect Immun. 2003 Oct;71(10):5682-9. doi: 10.1128/IAI.71.10.5682-5689.2003. Infect Immun. 2003. PMID: 14500489 Free PMC article.
-
Enterococcus Virulence and Resistant Traits Associated with Its Permanence in the Hospital Environment.Antibiotics (Basel). 2022 Jun 26;11(7):857. doi: 10.3390/antibiotics11070857. Antibiotics (Basel). 2022. PMID: 35884110 Free PMC article. Review.
-
Streptococcus gordonii pheromone s.g.cAM373 may influence the reservoir of antibiotic resistance determinants of Enterococcus faecalis origin in the oral metagenome.J Med Microbiol. 2017 Nov;66(11):1635-1639. doi: 10.1099/jmm.0.000613. Epub 2017 Oct 12. J Med Microbiol. 2017. PMID: 29022550 Free PMC article.
-
Cloning and expression of a novel lactococcal aggregation factor from Lactococcus lactis subsp. lactis BGKP1.BMC Microbiol. 2011 Dec 19;11:265. doi: 10.1186/1471-2180-11-265. BMC Microbiol. 2011. PMID: 22182285 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

