Genetic polymorphisms of group B streptococcus scpB alter functional activity of a cell-associated peptidase that inactivates C5a
- PMID: 10948119
- PMCID: PMC101725
- DOI: 10.1128/IAI.68.9.5018-5025.2000
Genetic polymorphisms of group B streptococcus scpB alter functional activity of a cell-associated peptidase that inactivates C5a
Abstract
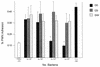
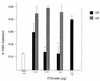
Many group B Streptococcus agalactiae strains and other pathogenic streptococci express a cell-associated peptidase that inactivates C5a (C5a-ase), the major neutrophil chemoattractant produced by activation of the complement cascade. Type III group B streptococci (GBS) can be classified genotypically into three restriction digest pattern types. Functional C5a-ase activity of GBS correlates with this genetic typing; therefore, we sought to identify a genetic basis for this phenomenon. Southern hybridization confirms that all type III GBS contain scpB, the gene encoding GBS C5a-ase. GBS strains with high C5a-ase functional activity and those with no or very low activity both express immunoreactive C5a-ase. The scpB sequence of strain I30, which has high C5a-ase activity, is 98.2% homologous to the previously reported serotype II GBS scpB sequence. The scpB sequences of strains I25 and GW, which have low or no C5a-ase activity, are identical. The predicted I25 and GW C5a-ase proteins share a four-amino-acid deletion affecting the protease histidine active-site consensus motif. Recombinant I30 C5a-ase has good functional activity, whereas recombinant I25 C5a-ase has low activity. These data demonstrate that functional C5a-ase differences between type III GBS strains are attributable to a genetic polymorphism of scpB. The ubiquitous expression of C5a-ase, irrespective of functional activity, suggests that C5a-ase may have a second, as yet unidentified, function.
Figures


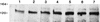




References
-
- Adderson E E, Shackelford P G, Quinn A, Carroll W L. Restricted IgH chain V gene usage in the human antibody response to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1991;147:1667–1674. - PubMed
-
- Bairoch A. Prosite. Geneva, Switzerland: Swiss Institute of Bioinformatics; 1999.
-
- Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: W. B. Saunders; 1995. pp. 980–1054.
-
- Bohnsack J F, Widjaja K, Ghazizadeh S, Rubens C E, Hillyard D, Parker C J, Albertine K H, Hill H R. A role for C5 in the acute neutrophil response to group B streptococcal infections. J Infect Dis. 1997;175:847–855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

