Effect of Helicobacter pylori on polymorphonuclear leukocyte migration across polarized T84 epithelial cell monolayers: role of vacuolating toxin VacA and cag pathogenicity island
- PMID: 10948148
- PMCID: PMC101782
- DOI: 10.1128/IAI.68.9.5225-5233.2000
Effect of Helicobacter pylori on polymorphonuclear leukocyte migration across polarized T84 epithelial cell monolayers: role of vacuolating toxin VacA and cag pathogenicity island
Abstract
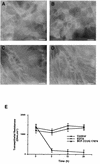
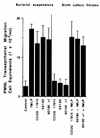
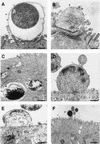
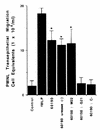
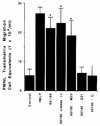
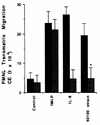
Helicobacter pylori infection can induce polymorphonuclear leukocyte (PMNL) infiltration of the gastric mucosa, which characterizes acute chronic gastritis. The mechanisms underlying this process are poorly documented. The lack of an in vitro model has considerably impaired the study of transepithelial migration of PMNL induced by H. pylori. In the present work, we used confluent polarized monolayers of the human intestinal cell line T84 grown on permeable filters to analyze the epithelial PMNL response induced by broth culture filtrates (BCFs) and bacterial suspensions from different strains of H. pylori. We have evaluated the role of the vacuolating cytotoxin VacA and of the cag pathogenicity island (PAI) of H. pylori in PMNL migration via their effects on T84 epithelial cells. We noted no difference in the rates of PMNL transepithelial migration after epithelial preincubation with bacterial suspensions or with BCFs of VacA-negative or VacA-positive H. pylori strains. In contrast, PMNL transepithelial migration was induced after incubation of the T84 cells with cag PAI-positive and cagE-positive H. pylori strains. Finally, PMNL migration was correlated with a basolateral secretion of interleukin-8 by T84 cells, thus creating a subepithelial chemotactic gradient for PMNL. These data provide evidence that the vacuolating cytotoxin VacA is not involved in PMNL transepithelial migration and that the cag PAI, with a pivotal role for the cagE gene, provokes a transcellular signal across T84 monolayers, inducing a subepithelial PMNL response.
Figures
Similar articles
-
Human polymorphonuclear leukocytes are sensitive in vitro to Helicobacter pylori vaca toxin.Helicobacter. 2006 Dec;11(6):544-55. doi: 10.1111/j.1523-5378.2006.00457.x. Helicobacter. 2006. PMID: 17083376
-
Epithelial intestinal cell apoptosis induced by Helicobacter pylori depends on expression of the cag pathogenicity island phenotype.Infect Immun. 2001 Aug;69(8):5001-9. doi: 10.1128/IAI.69.8.5001-5009.2001. Infect Immun. 2001. PMID: 11447179 Free PMC article.
-
The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells.Infect Immun. 2003 Mar;71(3):1068-74. doi: 10.1128/IAI.71.3.1068-1074.2003. Infect Immun. 2003. PMID: 12595416 Free PMC article.
-
[High prevalence of VacA, CagA protein expression and presence of cag pathogenicity island in Japanese Helicobacter pylori isolates].Nihon Rinsho. 1999 Jan;57(1):17-22. Nihon Rinsho. 1999. PMID: 10036931 Review. Japanese.
-
Virulence factors of Helicobacter pylori.Int J Med Microbiol. 2001 Mar;290(8):647-58. doi: 10.1016/s1438-4221(01)80002-3. Int J Med Microbiol. 2001. PMID: 11310443 Review.
Cited by
-
Impact of Helicobacter pylori on the healing process of the gastric barrier.World J Gastroenterol. 2016 Sep 7;22(33):7536-58. doi: 10.3748/wjg.v22.i33.7536. World J Gastroenterol. 2016. PMID: 27672275 Free PMC article.
-
Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells.Infect Immun. 2005 Jul;73(7):4180-9. doi: 10.1128/IAI.73.7.4180-4189.2005. Infect Immun. 2005. PMID: 15972508 Free PMC article.
-
Afa/Dr diffusely adhering Escherichia coli infection in T84 cell monolayers induces increased neutrophil transepithelial migration, which in turn promotes cytokine-dependent upregulation of decay-accelerating factor (CD55), the receptor for Afa/Dr adhesins.Infect Immun. 2003 Apr;71(4):1774-83. doi: 10.1128/IAI.71.4.1774-1783.2003. Infect Immun. 2003. PMID: 12654791 Free PMC article.
-
Investigation of the association between clinical outcome and the cag pathogenicity-island and other virulence genes of Helicobacter pylori isolates from patients with dyspepsia in Eastern Turkey.Braz J Microbiol. 2014 Mar 10;44(4):1267-74. doi: 10.1590/s1517-83822013000400034. eCollection 2013 Dec. Braz J Microbiol. 2014. PMID: 24688521 Free PMC article.
-
Impact of cagPAI and T4SS on the inflammatory response of human neutrophils to Helicobacter pylori infection.PLoS One. 2013 Jun 3;8(6):e64623. doi: 10.1371/journal.pone.0064623. Print 2014. PLoS One. 2013. PMID: 23755130 Free PMC article.
References
-
- Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek S E, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–54. - PubMed
-
- Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. - PubMed
-
- Baik S C, Youn H S, Chung M H, Lee W K, Cho M J, Ko G H, Park C K, Kasai H, Rhee K H. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 1996;56:1279–1282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

