Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading
- PMID: 10948167
- PMCID: PMC101801
- DOI: 10.1128/IAI.68.9.5377-5384.2000
Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading
Abstract
Burkholderia pseudomallei, a facultative intracellular bacterium, is the causative agent of a broad spectrum of diseases collectively known as melioidosis. Its ability to survive inside phagocytic and nonphagocytic cells and to induce multinucleated giant cell (MNGC) formation has been demonstrated. This study was designed to assess a possible mechanism(s) leading to this cellular change, using virulent and nonvirulent strains of B. pseudomallei to infect both phagocytic and nonphagocytic cell lines. We demonstrated that when the cells were labeled with two different cell markers (CMFDA or CMTMR), mixed, and then infected with B. pseudomallei, direct cell-to-cell fusion could be observed, leading to MNGC formation. Staining of the infected cells with rhodamine-conjugated phalloidin indicated that immediately after the infection, actin rearrangement into a comet tail appearance occurred, similar to that described earlier for other bacteria. The latter rearrangement led to the formation of bacterium-containing, actin-associated membrane protrusions which could lead to a direct cell-to-cell spreading of B. pseudomallei in the infected hosts. Results from 4', 6'-diamidine-2-phenylindole dihydrochloride (DAPI) nuclear staining, poly-ADP ribose polymerase cleavage, staining of infected cells for phosphatidylserine exposure with annexin V, and electrophoresis of the DNA extracted from these infected cells showed that B. pseudomallei could kill the host cells by inducing apoptosis in both phagocytic and nonphagocytic cells.
Figures
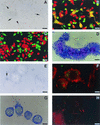

References
-
- Ahmed K, Enciso H D, Masaki H, Tao M, Omori A, Tharavichikul P, Nagatake T. Attachment of Burkholderia pseudomallei to pharyngeal epithelial cells. A highly pathogenic bacteria with low attachment ability. Am J Trop Med Hyg. 1999;60:90–93. - PubMed
-
- Chaowagul W, Suputtamongkol Y, Dance D A B, Rachanuvong A, Pattaraarechachai J, White N J. Relapse in melioidosis: incidence and risk factors. J Infect Dis. 1993;168:1181–1185. - PubMed
-
- Chaowagul W, White N J, Dance D A B, Wattanagoon Y, Naigowit P, Davis T M E, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890–899. - PubMed
-
- Cossart P, Boquet P, Normark S, Rappuoli R, editors. Cellular microbiology. Washington, D.C.: ASM Press; 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

