Prenylation of the floral transcription factor APETALA1 modulates its function
- PMID: 10948247
- PMCID: PMC149100
- DOI: 10.1105/tpc.12.8.1257
Prenylation of the floral transcription factor APETALA1 modulates its function
Abstract
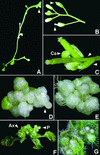
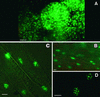
The Arabidopsis MADS box transcription factor APETALA1 (AP1) was identified as a substrate for farnesyltransferase and shown to be farnesylated efficiently both in vitro and in vivo. AP1 regulates the transition from inflorescence shoot to floral meristems and the development of sepals and petals. AP1 fused to green fluorescent protein (GFP) retained transcription factor activity and directed the expected terminal flower phenotype when ectopically expressed in transgenic Arabidopsis. However, ap1mS, a farnesyl cysteine-acceptor mutant of AP1, as well as the GFP-ap1mS fusion protein failed to direct the development of compound terminal flowers but instead induced novel phenotypes when ectopically expressed in Arabidopsis. Similarly, compound terminal flowers did not develop in era1-2 transformants that ectopically expressed AP1. Together, the results demonstrate that AP1 is a target of farnesyltransferase and suggest that farnesylation alters the function and perhaps specificity of the transcription factor.
Figures





References
-
- Anant, J.S., Ong, O.C., Xie, H., Clarke, S., O'Brein, P.J., and Fung, B.K.K. (1992). In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J. Biol. Chem. 267, 687–690. - PubMed
-
- Anderegg, R.J., Betz, R., Carr, S.A., Crabb, J.W., and Duntze, W. (1988). Structure of the Saccharomyces cerevisiae mating hormone a-factor: Identification of S-farnesyl cysteine as a structural component. J. Biol. Chem. 263, 18236–18240. - PubMed
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium–mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Ser. III 316, 1194–1199.
-
- Bent, A.F., Kunkel, B.N., Dahlbeck, D., Brown, K.L., Schmidt, R., Giraudat, J., Leung, J., and Staskawicz, B.J. (1994). RPS2 of Arabidopsis thaliana: A leucine-rich repeat class of plant disease resistance genes. Science 265, 1856–1860. - PubMed
-
- Bowman, J.L., Alvarez, J., Weigel, D., Meyerowitz, E.M., and Smyth, D.R. (1993). Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119, 721–743.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

