Pea compound leaf architecture is regulated by interactions among the genes UNIFOLIATA, cochleata, afila, and tendril-lessn
- PMID: 10948249
- PMCID: PMC149102
- DOI: 10.1105/tpc.12.8.1279
Pea compound leaf architecture is regulated by interactions among the genes UNIFOLIATA, cochleata, afila, and tendril-lessn
Abstract
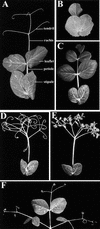
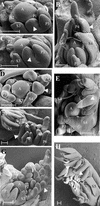
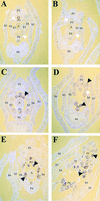
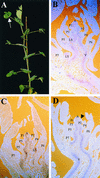
The compound leaf primordium of pea represents a marginal blastozone that initiates organ primordia, in an acropetal manner, from its growing distal region. The UNIFOLIATA (UNI) gene is important in marginal blastozone maintenance because loss or reduction of its function results in uni mutant leaves of reduced complexity. In this study, we show that UNI is expressed in the leaf blastozone over the period in which organ primordia are initiated and is downregulated at the time of leaf primordium determination. Prolonged UNI expression was associated with increased blastozone activity in the complex leaves of afila (af), cochleata (coch), and afila tendril-less (af tl) mutant plants. Our analysis suggests that UNI expression is negatively regulated by COCH in stipule primordia, by AF in proximal leaflet primordia, and by AF and TL in distal and terminal tendril primordia. We propose that the control of UNI expression by AF, TL, and COCH is important in the regulation of blastozone activity and pattern formation in the compound leaf primordium of the pea.
Figures




Similar articles
-
Regulation of stipule development by COCHLEATA and STIPULE-REDUCED genes in pea Pisum sativum.Planta. 2009 Aug;230(3):449-58. doi: 10.1007/s00425-009-0952-0. Epub 2009 Jun 2. Planta. 2009. PMID: 19488780
-
Effects of MULTIFOLIATE-PINNA, AFILA, TENDRIL-LESS and UNIFOLIATA genes on leafblade architecture in Pisum sativum.Planta. 2009 Jun;230(1):177-90. doi: 10.1007/s00425-009-0931-5. Epub 2009 Apr 29. Planta. 2009. PMID: 19404676
-
Unifoliata-Afila interactions in pea leaf morphogenesis.Am J Bot. 2013 Mar;100(3):478-95. doi: 10.3732/ajb.1200611. Epub 2013 Feb 10. Am J Bot. 2013. PMID: 23400494
-
Functional Genomics and Genetic Control of Compound Leaf Development in Medicago truncatula: An Overview.Methods Mol Biol. 2018;1822:197-203. doi: 10.1007/978-1-4939-8633-0_14. Methods Mol Biol. 2018. PMID: 30043306 Review.
-
Coordination of leaf development via regulation of KNOX1 genes.J Plant Res. 2010 Jan;123(1):7-14. doi: 10.1007/s10265-009-0248-2. Epub 2009 Jun 9. J Plant Res. 2010. PMID: 19506991 Review.
Cited by
-
The diverse roles of cytokinins in regulating leaf development.Hortic Res. 2021 Jun 1;8(1):118. doi: 10.1038/s41438-021-00558-3. Hortic Res. 2021. PMID: 34059666 Free PMC article. Review.
-
The mutant crispa reveals multiple roles for PHANTASTICA in pea compound leaf development.Plant Cell. 2005 Apr;17(4):1046-60. doi: 10.1105/tpc.104.029447. Epub 2005 Mar 4. Plant Cell. 2005. PMID: 15749758 Free PMC article.
-
PROLIFERATING INFLORESCENCE MERISTEM, a MADS-box gene that regulates floral meristem identity in pea.Plant Physiol. 2002 Jul;129(3):1150-9. doi: 10.1104/pp.001677. Plant Physiol. 2002. PMID: 12114569 Free PMC article.
-
MtNODULE ROOT1 and MtNODULE ROOT2 Are Essential for Indeterminate Nodule Identity.Plant Physiol. 2018 Sep;178(1):295-316. doi: 10.1104/pp.18.00610. Epub 2018 Jul 19. Plant Physiol. 2018. PMID: 30026291 Free PMC article.
-
A molecular framework underlying the compound leaf pattern of Medicago truncatula.Nat Plants. 2020 May;6(5):511-521. doi: 10.1038/s41477-020-0642-2. Epub 2020 May 11. Nat Plants. 2020. PMID: 32393879
References
-
- Angiosperm Phylogeny Group. (1998). An ordinal classification for the families of flowering plants. Ann. Mo. Bot. Gard. 85, 531–553.
-
- Anthony, R.G., James, P.E., and Jordan, B.R. (1993). Cloning and sequence analysis of a flo/lfy homologue isolated from cauliflower (Brassica oleracea L. var. botrytis). Plant Mol. Biol. 22, 1163–1166. - PubMed
-
- Blázquez, M.A., and Weigel, D. (2000). Integration of floral inductive signals in Arabidopsis. Nature 404, 889–892. - PubMed
-
- Blázquez, M.A., Soowal, L.N., Lee, I., and Weigel, D. (1997). LEAFY expression and flower initiation in Arabidopsis. Development 124, 3835–3844. - PubMed
-
- Blixt, S. (1967). Linkage studies in Pisum VII. The manifestation of the genes cri, and coch, and the double-recessive in Pisum. Agric. Hort. Genet. 25, 131–144.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

