Wound-induced expression and activation of WIG, a novel glycogen synthase kinase 3
- PMID: 10948263
- PMCID: PMC149116
- DOI: 10.1105/tpc.12.8.1467
Wound-induced expression and activation of WIG, a novel glycogen synthase kinase 3
Abstract
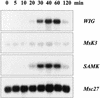
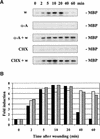
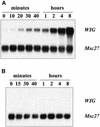
Glycogen synthase kinase 3 (GSK-3) is involved in the regulation of several physiological processes, including glycogen metabolism, protein synthesis, transcription factor activity, and developmental control. Although GSK-3-like genes have been isolated from plants, no function for any of these kinases has been defined. We report here that the alfalfa wound-induced gene (WIG, for wound-induced GSK-3), lencoding a functional plant GSK-3-like kinase, is activated when the alfalfa leaves are wounded. Although WIG transcripts are hardly detectable in mature leaves, WIG mRNA accumulates rapidly after wounding. Using a peptide antibody that specifically recognizes p53(WIG), we show that p53(WIG) kinase is activated immediately after wounding. Wound-induced activation of p53(WIG) kinase is a post-translational process, because the concentrations of p53(WIG) protein do not change in intact and wounded leaves, and inhibition of transcription or translation does not block activation by wounding. However, inactivation of p53(WIG) kinase, which usually occurs within 60 min after wounding, is dependent on transcription and translation of one or more protein factors. These data suggest that the WIG kinase is involved in wound signaling in plants.
Figures



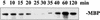
References
-
- Alessi, D.R., and Cohen, P. (1998). Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 8, 55–62. - PubMed
-
- Anderton, B.H. (1999). Alzheimer's disease: Clues from flies and worms. Curr. Biol. 9, R106–R109. - PubMed
-
- Baldwin, I.T., and Preston, C.A. (1999). The eco-physiological complexity of plant responses to insect herbivores. Planta 208, 137–145.
-
- Bianchi, M.W., Guivarc'h, D., Thomas, M., Woodgett, J.R., and Kreis, M. (1994). Arabidopsis homologs of the shaggy and GSK-3 protein kinases: Molecular cloning and functional expression in Escherichia coli. Mol. Gen. Genet. 242, 337–345. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

