Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar
- PMID: 10950871
- PMCID: PMC316855
Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar
Abstract
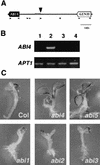
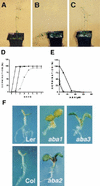
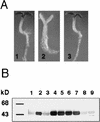
Sugars have signaling roles in a wide variety of developmental processes in plants. To elucidate the regulatory components that constitute the glucose signaling network governing plant growth and development, we have isolated and characterized two Arabidopsis glucose insensitive mutants, gin5 and gin6, based on a glucose-induced developmental arrest during early seedling morphogenesis. The T-DNA-tagged gin6 mutant abrogates the glucose-induced expression of a putative transcription factor, ABI4, previously shown to be involved in seed-specific abscisic acid (ABA) responses. Thus, ABI4 might be a regulator involved in both glucose- and seed-specific ABA signaling. The characterization of the gin5 mutant, on the other hand, reveals that glucose-specific accumulation of ABA is essential for hexokinase-mediated glucose responses. Consistent with this result, we show that three ABA-deficient mutants (aba1-1, aba2-1, and aba3-2) are also glucose insensitive. Exogenous ABA can restore normal glucose responses in gin5 and aba mutants but not in gin6 plants. Surprisingly, only abi4 and abi5-1 but not other ABA-insensitive signaling mutants (abi1-1, abi2-1, and abi3-1) exhibit glucose insensitivity, indicating the involvement of a distinct ABA signaling pathway in glucose responses. These results provide the first direct evidence to support a novel and central role of ABA in plant glucose responses mediated through glucose regulation of both ABA levels by GIN5 and ABA signaling by GIN6/ABI4.
Figures




References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York, NY: John Wiley & Sons; 1987.
-
- Bell CJ, Ecker JR. Assignement of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. - PubMed
-
- Bonetta D, McCourt P. Genetic analysis of ABA signal transduction pathways. Trends Plant Sci. 1998;3:231–235.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
