Delayed wound healing in CXCR2 knockout mice
- PMID: 10951241
- PMCID: PMC2664868
- DOI: 10.1046/j.1523-1747.2000.00034.x
Delayed wound healing in CXCR2 knockout mice
Erratum in
- J Invest Dermatol 2000 Nov;115(5):931
Abstract
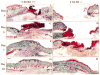
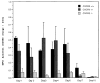
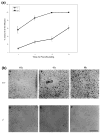
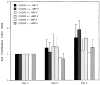
Previous studies demonstrated that the CXC chemokine, MGSA/GRO-alpha and its receptor, CXCR2, are expressed during wound healing by keratinocytes and endothelial cells at areas where epithelialization and neovascularization occur. The process of wound healing is dependent on leukocyte recruitment, keratinocyte proliferation and migration, and angiogenesis. These processes may be mediated in part by CXC chemokines, such as interleukin-8 and MGSA/GRO-alpha. To examine further the significance of CXC chemokines in wound healing, full excisional wounds were created on CXCR2 wild-type (+/+), heterozygous (+/-), or knockout (-/-) mice. Wounds were histologically analyzed for neutrophil and monocyte infiltration, neovascularization and epithelialization at days 3, 5, 7, and 10 postwounding. The CXCR2 -/- mice exhibited defective neutrophil recruitment, an altered temporal pattern of monocyte recruitment, and altered secretion of interleukin-1beta. Significant delays in wound healing parameters, including epithelialization and decreased neovascularization, were also observed in CXCR2 -/- mice. In vitro wounding experiments with cultures of keratinocytes established from -/- and +/+ mice revealed a retardation in wound closure in CXCR2 -/- keratinocytes, suggesting a role for this receptor on keratinocytes in epithelial resurfacing that is independent of neutrophil recruitment. These in vitro and in vivo studies further establish a pathophysiologic role for CXCR2 during cutaneous wound repair.
Figures

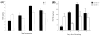
References
-
- Abbot RE, Corral CJ, Maclvor DM, Lin X, Ley TJ, Mustoe TA. Augmented inflammatory response and altered wound healing in cathepsin G-deficient mice. Arch Surg. 1998;133:1002–1006. - PubMed
-
- Addison CL, Daniel TO, Ehlert JE, et al. The CXC. chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine induced angiogenic activity. J Immunol. accepted. - PubMed
-
- Brauchle M, Fassler R, Werner S. Suppression of keratinocyte growth factor expression by glucocorticoids in vitro and during wound healing. J Invest Dermatol. 1995;105:579–584. - PubMed
-
- Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

