Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50
- PMID: 10952568
- PMCID: PMC90058
- DOI: 10.1128/AAC.44.9.2276-2285.2000
Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50
Abstract
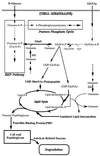
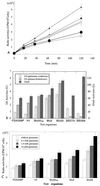
Staphylococcus aureus Mu50, which has reduced susceptibility to vancomycin, has a remarkably thickened cell wall with an increased proportion of glutamine nonamidated muropeptides. In addition, Mu50 had enhanced glutamine synthetase and L-glutamine D-fructose-6-phosphate aminotransferase activities, which are involved in the cell-wall peptidoglycan synthesis pathway. Furthermore, significantly increased levels of incorporation of (14)C-labeled D-glucose into the cell wall was observed in Mu50. Unlike a femC mutant S. aureus strain, increased levels of production of nonamidated muropeptides in Mu50 was not caused by lower levels of glutamine synthetase activity but was considered to be due to the glutamine depletion caused by increased glucose utilization by the cell to biosynthesize increased amounts of peptidoglycan. After the cells were allowed to synthesize cell wall in the absence or presence of glucose and glutamine, cells with different cell-wall thicknesses and with cell walls with different levels of cross-linking were prepared, and susceptibility testing of these cells demonstrated a strong correlation between the cell-wall thickness and the degree of vancomycin resistance. Affinity trapping of vancomycin molecules by the cell wall and clogging of the outer layers of peptidoglycan by bound vancomycin molecules were considered to be the mechanism of vancomycin resistance of Mu50. The reduced cross-linking and the increased affinity of binding to vancomycin of the Mu50 cell wall presumably caused by the increased proportion of nonamidated muropeptides may also contribute to the resistance to some extent.
Figures





References
-
- Bearne S L. Active site-directed inactivation of Escherichia coli glucosamine-6-phosphate synthase. Determination of the fructose 6-phosphate binding constant using a carbohydrate-based inactivator. J Biol Chem. 1996;271:3052–3057. - PubMed
-
- Ghosh S, Blumenthal H J, Davidson E, Roseman S. Glucosamine metabolism. V. Enzymatic synthesis of glucosamine-6-phosphate. J Biol Chem. 1960;235:1265–1273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

