Intestinal anti-inflammatory activity of UR-12746, a novel 5-ASA conjugate, on acute and chronic experimental colitis in the rat
- PMID: 10952687
- PMCID: PMC1572265
- DOI: 10.1038/sj.bjp.0703505
Intestinal anti-inflammatory activity of UR-12746, a novel 5-ASA conjugate, on acute and chronic experimental colitis in the rat
Abstract
The present study was undertaken to investigate the intestinal anti-inflammatory effects of UR-12746 on the acute and chronic stages of a trinitrobenzene sulphonic acid (TNBS) experimental model of inflammatory bowel disease (IBD) in the rat. UR-12746 is a novel, locally-acting compound which combines, through an azo bond, 5-aminosalicylic (5-ASA) and UR-12715, a potent platelet activating factor (PAF)-antagonist. UR-12746 oral pretreatment of colitic rats (50 and 100 mg kg(-1)) reduced acute colonic damage when evaluated 2 days after colonic insult. Postreatment for 4 weeks with UR-12746 (50 and 100 mg kg(-1)) resulted in a faster recovery of the damaged colonic mucosa, which was macroscopically significant from the third week. The intestinal anti-inflammatory effect of UR-12746 was associated with a decrease in leukocyte infiltration in the colonic mucosa, which was evidenced both biochemically, by a reduction in myeloperoxidase activity, and histologically, by a lower leukocyte count after morphometric analysis. This effect was higher than that seen with sulphasalazine, when assayed at the same doses and in the same experimental conditions. Several mechanisms can be involved in the beneficial effects showed by UR-12746: inhibition of leukotriene B(4) synthesis in the inflamed colon, improvement of the altered colonic oxidative status, and reduction of colonic interleukin-1beta production. The results suggest that the intestinal anti-inflammatory activity of UR-12746 can be attributed to the additive effects exerted by 5-ASA and UR-12715, the PAF antagonist compound, that are released in the colonic lumen after reduction of the azo bond by the intestinal bacteria.
Figures



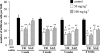

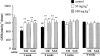
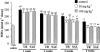

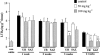
References
-
- ANDERSON M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. - PubMed
-
- ASFAHA S., BELL C.J., WALLACE J.L., MACNAUGHTON W.K. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am. J. Physiol. 1999;276:G703–G710. - PubMed
-
- BARANES J., HELLEGOUARCH A., LE HEGARAT M., VIOSSAT I., AUGUET M., CHABRIER P., BRAQUET F., BRAQUET P. The effects of PAF-acether on the cardiovascular system and their inhibition by a new highly specific PAF-acether receptor antagonist BN-52021. Pharmacol. Res. Commun. 1986;18:717–737. - PubMed
-
- BELL C.J., GALL D.G., WALLACE J.L. Disruption of colonic electrolyte transport in experimental colitis. Am. J. Physiol. 1995;268:G622–G630. - PubMed
-
- CASALS-STENZEL J., MUACEVIC G., WEBER K. Pharmacological actions of WEB-2086, a new specific antagonist of platelet-activating factor. J. Pharmacol. Exp. Ther. 1987;241:974–981. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

