Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions
- PMID: 10952719
- PMCID: PMC2193239
- DOI: 10.1084/jem.192.4.495
Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions
Abstract
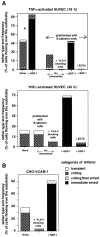
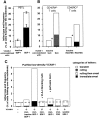
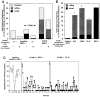
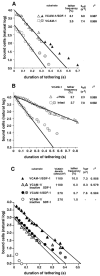
Leukocyte recruitment to target tissue is initiated by weak rolling attachments to vessel wall ligands followed by firm integrin-dependent arrest triggered by endothelial chemokines. We show here that immobilized chemokines can augment not only arrest but also earlier integrin-mediated capture (tethering) of lymphocytes on inflamed endothelium. Furthermore, when presented in juxtaposition to vascular cell adhesion molecule 1 (VCAM-1), the endothelial ligand for the integrin very late antigen 4 (VLA-4, alpha4beta1), chemokines rapidly augment reversible lymphocyte tethering and rolling adhesions on VCAM-1. Chemokines potentiate VLA-4 tethering within <0.1 s of contact through Gi protein signaling, the fastest inside-out integrin signaling events reported to date. Although VLA-4 affinity is not altered upon chemokine signaling, subsecond VLA-4 clustering at the leukocyte-substrate contact zone results in enhanced leukocyte avidity to VCAM-1. Endothelial chemokines thus regulate all steps in adhesive cascades that control leukocyte recruitment at specific vascular beds.
Figures

References
-
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
-
- Kim C.H., Broxmeyer H.E. Chemokinessignal lamps for trafficking of T and B cells for development and effector function. J. Leukoc. Biol. 1999;65:6–15. - PubMed
-
- Campbell J.J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P., Andrew D.P., Warnke R., Ruffing N., Kassam N. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

