Macrophage-tropic HIV induces and exploits dendritic cell chemotaxis
- PMID: 10952729
- PMCID: PMC2193233
- DOI: 10.1084/jem.192.4.587
Macrophage-tropic HIV induces and exploits dendritic cell chemotaxis
Abstract
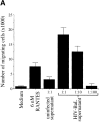
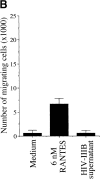
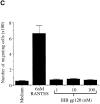
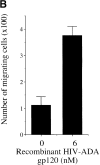
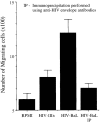
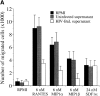
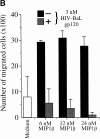
Immature dendritic cells (iDCs) express the CC chemokine receptor (CCR)5, which promotes chemotaxis toward the CC chemokines regulated on activation, normal T cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1alpha, and MIP-1beta. By contrast, mature DCs downregulate CCR5 but upregulate CXC chemokine receptor (CXCR)4, and as a result exhibit enhanced chemotaxis toward stromal cell-derived factor (SDF)-1alpha. CCR5 and CXCR4 also function as coreceptors for macrophage-tropic (M-tropic) and T cell-tropic (T-tropic) human immunodeficiency virus (HIV)-1, respectively. Here, we demonstrate chemotaxis of iDCs toward M-tropic (R5) but not T-tropic (X4) HIV-1. Furthermore, preexposure to M-tropic HIV-1 or its recombinant envelope protein prevents migration toward CCR5 ligands. The migration of iDCs toward M-tropic HIV-1 may enhance formation of DC-T cell syncytia, thus promoting viral production and destruction of both DC and T helper lymphocytes. Therefore, disturbance of DC chemotaxis by HIV-1 is likely to contribute to immunosuppression in primary infection and AIDS. In addition, migration of iDCs toward HIV-1 may aid the capture of R5 HIV-1 virions by the abundant DC cell surface protein DC-specific intercellular adhesion molecule (ICAM)3-grabbing nonintegrin (DC-SIGN). HIV-1 bound to DC cell-specific DC-SIGN retains the ability to infect replication-permissive T cells in trans for several days. Consequently, recruitment of DC by HIV-1 could combine with the ability of DC-SIGN to capture and transmit the virus to T cells, and so facilitate dissemination of virus within an infected individual.
Figures


References
-
- Knight S.C., Patterson S. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu. Rev. Immunol. 1997;15:593–615. - PubMed
-
- Cameron P.U., Freudenthal P.S., Barker J.M., Gezelter S., Inaba K., Steinman R.M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

