Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilized human neutrophils
- PMID: 10953003
- PMCID: PMC2175292
- DOI: 10.1083/jcb.150.4.785
Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilized human neutrophils
Abstract
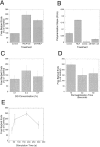
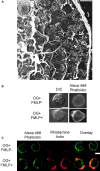
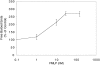
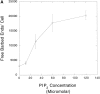
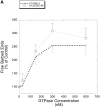
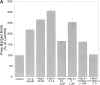
We developed a permeabilization method that retains coupling between N-formyl-methionyl-leucyl-phenylalanine tripeptide (FMLP) receptor stimulation, shape changes, and barbed-end actin nucleation in human neutrophils. Using GTP analogues, phosphoinositides, a phosphoinositide-binding peptide, constitutively active or inactive Rho GTPase mutants, and activating or inhibitory peptides derived from neural Wiskott-Aldrich syndrome family proteins (N-WASP), we identified signaling pathways leading from the FMLP receptor to actin nucleation that require Cdc42, but then diverge. One branch traverses the actin nucleation pathway involving N-WASP and the Arp2/3 complex, whereas the other operates through active Rac to promote actin nucleation. Both pathways depend on phosphoinositide expression. Since maximal inhibition of the Arp2/3 pathway leaves an N17Rac inhibitable alternate pathway intact, we conclude that this alternate involves phosphoinositide-mediated uncapping of actin filament barbed ends.
Figures







References
-
- Akasaki T., Koga H., Sumimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J. Biol. Chem. 1999;274:18055–18059. - PubMed
-
- Amrein P.C., Stossel T.P. Prevention of degradation of human polymorphonuclear leukocyte proteins by diisopropylfluorophosphate. Blood. 1980;56:442–447. - PubMed
-
- Arcaro A. The small GTP-binding protein Rac promotes the dissociation of gelsolin from actin filaments in neutrophils. J. Biol. Chem. 1998;273:805–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

