Stimulation of fascin spikes by thrombospondin-1 is mediated by the GTPases Rac and Cdc42
- PMID: 10953005
- PMCID: PMC2175285
- DOI: 10.1083/jcb.150.4.807
Stimulation of fascin spikes by thrombospondin-1 is mediated by the GTPases Rac and Cdc42
Abstract
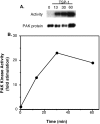
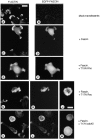
Cell adhesion to extracellular matrix is an important physiological stimulus for organization of the actin-based cytoskeleton. Adhesion to the matrix glycoprotein thrombospondin-1 (TSP-1) triggers the sustained formation of F-actin microspikes that contain the actin-bundling protein fascin. These structures are also implicated in cell migration, which may be an important function of TSP-1 in tissue remodelling and wound repair. To further understand the function of fascin microspikes, we examined whether their assembly is regulated by Rho family GTPases. We report that expression of constitutively active mutants of Rac or Cdc42 triggered localization of fascin to lamellipodia, filopodia, and cell edges in fibroblasts or myoblasts. Biochemical assays demonstrated prolonged activation of Rac and Cdc42 in C2C12 cells adherent to TSP-1 and activation of the downstream kinase p21-activated kinase (PAK). Expression of dominant-negative Rac or Cdc42 in C2C12 myoblasts blocked spreading and formation of fascin spikes on TSP-1. Spreading and spike assembly were also blocked by pharmacological inhibition of F-actin turnover. Shear-loading of monospecific anti-fascin immunoglobulins, which block the binding of fascin to actin into cytoplasm, strongly inhibited spreading, actin cytoskeletal organization and migration on TSP-1 and also affected the motility of cells on fibronectin. We conclude that fascin is a critical component downstream of Rac and Cdc42 that is needed for actin cytoskeletal organization and cell migration responses to thrombospondin-1.
Figures
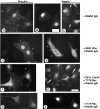
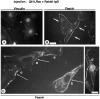
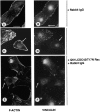
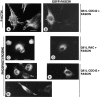
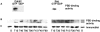
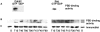






Comment in
-
Rho GTPases. Integrating integrin signaling.J Cell Biol. 2000 Aug 21;150(4):F107-9. doi: 10.1083/jcb.150.4.f107. J Cell Biol. 2000. PMID: 10953018 Free PMC article. No abstract available.
References
-
- Adams J.C. Formation of stable microspikes containing actin and the 55kDa actin-bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1implications for the antiadhesive activities of thrombospondin-1. J. Cell Sci. 1995;108:1977–1990. - PubMed
-
- Adams J.C., Tucker R.P., Lawler J. The thrombospondin gene family 1995. R.G. Landes; Austin, TX: pp. 188
-
- Arber S., Barbayannis F.A., Hanser H., Schneider C., Stanyon C.A., Bernard O., Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

