Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis
- PMID: 10953012
- PMCID: PMC2175271
- DOI: 10.1083/jcb.150.4.887
Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis
Abstract
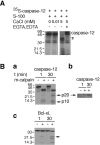
Calpains and caspases are two cysteine protease families that play important roles in regulating pathological cell death. Here, we report that m-calpain may be responsible for cleaving procaspase-12, a caspase localized in the ER, to generate active caspase-12. In addition, calpain may be responsible for cleaving the loop region in Bcl-xL and, therefore, turning an antiapoptotic molecule into a proapoptotic molecule. We propose that disturbance to intracellular calcium storage as a result of ischemic injury or amyloid beta peptide cytotoxicity may induce apoptosis through calpain- mediated caspase-12 activation and Bcl-xL inactivation. These data suggest a novel apoptotic pathway involving calcium-mediated calpain activation and cross-talks between calpain and caspase families.
Figures

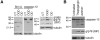
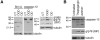
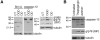
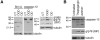

References
-
- Alzheimer's Disease Collaborative Group The structure of the presenilin 1 (S182) gene and identification of six novel mutations in early onset AD families. Nat. Genet. 1995;11:219–222. - PubMed
-
- Begley J.G., Duan W., Chan S., Duff K., Mattson M.P. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J. Neurochem. 1999;72:1030–1039. - PubMed
-
- Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Berridge M.J., Bootman M.D., Lipp P. Calciuma life and death signal. Nature. 1998;395:645–648. - PubMed
-
- Bialik S., Cryns V.L., Drincic A., Miyata S., Wollowick A.L., Srinivasan A., Kitsis R.N. The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ. Res. 1999;85:403–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

