Tenascin-C suppresses Rho activation
- PMID: 10953015
- PMCID: PMC2175281
- DOI: 10.1083/jcb.150.4.913
Tenascin-C suppresses Rho activation
Abstract
Cell binding to extracellular matrix (ECM) components changes cytoskeletal organization by the activation of Rho family GTPases. Tenascin-C, a developmentally regulated matrix protein, modulates cellular responses to other matrix proteins, such as fibronectin (FN). Here, we report that tenascin-C markedly altered cell phenotype on a three-dimensional fibrin matrix containing FN, resulting in suppression of actin stress fibers and induction of actin-rich filopodia. This distinct morphology was associated with complete suppression of the activation of RhoA, a small GTPase that induces actin stress fiber formation. Enforced activation of RhoA circumvented the effects of tenascin. Effects of active Rho were reversed by a Rho inhibitor C3 transferase. Suppression of GTPase activation allows tenascin-C expression to act as a regulatory switch to reverse the effects of adhesive proteins on Rho function. This represents a novel paradigm for the regulation of cytoskeletal organization by ECM.
Figures
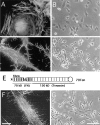
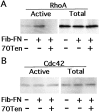
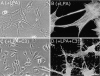
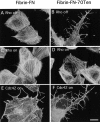
Comment in
-
Rho GTPases. Integrating integrin signaling.J Cell Biol. 2000 Aug 21;150(4):F107-9. doi: 10.1083/jcb.150.4.f107. J Cell Biol. 2000. PMID: 10953018 Free PMC article. No abstract available.
References
-
- Adams J.C., Watt F.M. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
-
- Aukhil I., Joshi P., Yan Y., Erickson H.P. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J. Biol. Chem. 1993;268:2542–2553. - PubMed
-
- Aukhil I., Slemp C.A., Lightner V.A., Nishimura K., Briscoe G., Erickson H.P. Purification of hexabrachion (tenascin) from cell culture conditioned medium and separation from a cell adhesion factor. Matrix. 1990;10:98–111. - PubMed
-
- Bagrodia S., Derijard B., Davis R.J., Cerione R.A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 1995;270:27995–27998. - PubMed
-
- Benard V., Bohl B.P., Bokoch G.M. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 1999;274:13198–13204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

