Insights into insulin resistance and type 2 diabetes from knockout mouse models
- PMID: 10953020
- PMCID: PMC380257
- DOI: 10.1172/JCI10830
Insights into insulin resistance and type 2 diabetes from knockout mouse models
Figures
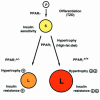

Comment in
-
Unraveling the mechanism of action of thiazolidinediones.J Clin Invest. 2000 Dec;106(11):1305-7. doi: 10.1172/JCI11705. J Clin Invest. 2000. PMID: 11104782 Free PMC article. No abstract available.
References
-
- Kadowaki T, et al. Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia. 1984;26:44–49. - PubMed
-
- Taylor SI, Accili D, Imai Y. Insulin resistance or insulin deficiency: which is the primary cause of NIDDM? Diabetes. 1994;43:735–740. - PubMed
-
- Moller DE. Transgenic approaches to the pathogenesis of NIDDM. Diabetes. 1994;43:1394–1401. - PubMed
-
- Accili D, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

