Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia
- PMID: 10953023
- PMCID: PMC380245
- DOI: 10.1172/JCI8342
Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia
Abstract
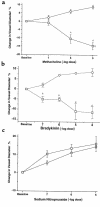
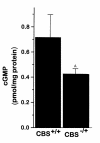
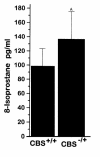
Homocysteine is a risk factor for the development of atherosclerosis and its thrombotic complications. We have employed an animal model to explore the hypothesis that an increase in reactive oxygen species and a subsequent loss of nitric oxide bioactivity contribute to endothelial dysfunction in mild hyperhomocysteinemia. We examined endothelial function and in vivo oxidant burden in mice heterozygous for a deletion in the cystathionine beta-synthase (CBS) gene, by studying isolated, precontracted aortic rings and mesenteric arterioles in situ. CBS(-/+) mice demonstrated impaired acetylcholine-induced aortic relaxation and a paradoxical vasoconstriction of mesenteric microvessels in response to superfusion of methacholine and bradykinin. Cyclic GMP accumulation following acetylcholine treatment was also impaired in isolated aortic segments from CBS(-/+) mice, but aortic relaxation and mesenteric arteriolar dilation in response to sodium nitroprusside were similar to wild-type. Plasma levels of 8-epi-PGF(2alpha) (8-IP) were somewhat increased in CBS(-/+) mice, but liver levels of 8-IP and phospholipid hydroperoxides, another marker of oxidative stress, were normal. Aortic tissue from CBS(-/+) mice also demonstrated greater superoxide production and greater immunostaining for 3-nitrotyrosine, particularly on the endothelial surface. Importantly, endothelial dysfunction appears early in CBS(-/+) mice in the absence of structural arterial abnormalities. Hence, mild hyperhomocysteinemia due to reduced CBS expression impairs endothelium-dependent vasodilation, likely due to impaired nitric oxide bioactivity, and increased oxidative stress apparently contributes to inactivating nitric oxide in chronic, mild hyperhomocysteinemia.
Figures

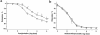


References
-
- Clarke R, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. - PubMed
-
- Stampfer MJ, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA. 1992;268:877–881. - PubMed
-
- Boers GH, Smals AG, Trijbels FJ, Fowler B, Bakkeren JA. Heterozygosity for homocystinuria in premature peripheral and cerebral occlusive arterial disease. N Engl J Med. 1985;313:709–715. - PubMed
-
- Selhub J, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–291. - PubMed
-
- Harker LA, Harlan JM, Ross R. Effect of sulfinpyrazone on homocysteine-induced endothelial injury and arteriosclerosis in baboons. Circ Res. 1983;53:731–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

