Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice
- PMID: 10953032
- PMCID: PMC380249
- DOI: 10.1172/JCI9087
Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice
Abstract
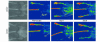
Endothelial cell progenitors, angioblasts, have been detected in the peripheral blood of adult humans, mice, and rabbits. These cells have been shown to incorporate into the endothelium of newly forming blood vessels in pathological and nonpathological conditions. Here we investigated the possibility that the CD34-expressing leukocytes (CD34(+) cells) that appear to be enriched for angioblasts could be used to accelerate the rate of blood-flow restoration in nondiabetic and diabetic mice undergoing neovascularization due to hindlimb ischemia. CD34(+) cells did not accelerate the restoration of flow in nondiabetic mice, but dramatically increased it in diabetic mice. Furthermore, CD34(+) cells derived from type 1 diabetics produced fewer differentiated endothelial cells in culture than did their type 2 diabetic- or nondiabetic-derived counterparts. In vitro experiments suggest that hyperglycemia per se does not alter the ability of angioblasts to differentiate or of angioblast-derived endothelial cells to proliferate. In contrast, hyperinsulinemia may enhance angioblast differentiation but impair angioblast-derived endothelial cell survival or proliferation. Our findings suggest that CD34(+) cells may be a useful tool for therapeutic angiogenesis in diabetics.
Figures




References
-
- Hueper WC, Russell MA. “Capillary-like formations” in tissue cultures of leukocytes. Archiv für Experimentelle Zellforschung Besoners Gewebezüchtung. 1932;12:407–424.
-
- Parker RC. The development of organized vessels in cultures of blood cells. Science. 1933;77:544–546. - PubMed
-
- White JF, Parshley MS. Growth in vitro of blood vessels from bone marrow of adult chickens. Am J Anat. 1950;89:321–345. - PubMed
-
- Hladovec J, Rossmann P. Circulating endothelial cells isolated together with platelets and the experimental modification of their counts in rats. Thromb Res. 1973;3:665–674.
-
- Solovey A, et al. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337:1584–1590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

