In vivo genomic footprinting of the human T-cell leukemia virus type 1 (HTLV-1) long terminal repeat enhancer sequences in HTLV-1-infected human T-cell lines with different levels of Tax I activity
- PMID: 10954525
- PMCID: PMC116336
- DOI: 10.1128/jvi.74.18.8277-8285.2000
In vivo genomic footprinting of the human T-cell leukemia virus type 1 (HTLV-1) long terminal repeat enhancer sequences in HTLV-1-infected human T-cell lines with different levels of Tax I activity
Abstract
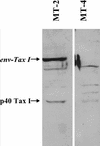
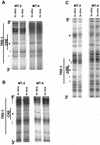
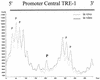
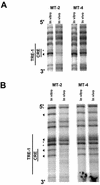
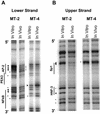
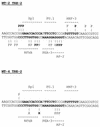
The Tax protein of human T-cell leukemia virus type 1 (HTLV-1) enhances viral gene expression through sequences in the U3 region of the viral long terminal repeat. These sequences consist of three imperfect 21-bp repeats (TRE-1s) and a region between the promoter-central and promoter-proximal 21-bp repeats (TRE-2). The TRE-1s contain a core cyclic AMP response element (CRE) motif and can be bound by CREB, ATF-1, ATF-2, and other members of the CREB-ATF superfamily of transcription factors. Tax enhances CREB binding to TRE-1 in vitro, and it promotes dimerization of CREB as well as other bZIP proteins. Using ligation-mediated PCR on in vivo dimethyl sulfate-treated HTLV-1-infected cell lines MT-2 and MT-4, we have compiled a profile of protein occupancy in the HTLV-1 enhancer sequences in the presence of high (MT-2) and low (MT-4) levels of biologically active Tax I. The in vivo footprinting showed that all three TRE-1s were bound by protein(s), but only in MT-2 cells. In MT-2 cells, all TRE-1s showed strong protection of the G residues in the central CRE, and the footprints extended to differing degrees into the GC-rich flanking sequences. This indicated Tax I-dependent loading of transcription factors onto the HTLV-1 TRE-1s in vivo. In vivo footprinting on TRE-2 indicated that this region was bound by proteins regardless of the Tax I status of the cell line. However, the presence of Tax I increased the extent and altered the profile of proteins binding TRE-2 in vivo.
Figures


References
-
- Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-1 tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652–1655. - PubMed
-
- Baranger A M, Palmer C R, Hamm M K, Giebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA-binding enhancement by the human T-cell leukaemia virus transactivator Tax. Nature. 1995;376:606–608. - PubMed
-
- Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

