Phosphorylation of simian virus 40 T antigen on Thr 124 selectively promotes double-hexamer formation on subfragments of the viral core origin
- PMID: 10954562
- PMCID: PMC116373
- DOI: 10.1128/jvi.74.18.8601-8613.2000
Phosphorylation of simian virus 40 T antigen on Thr 124 selectively promotes double-hexamer formation on subfragments of the viral core origin
Abstract
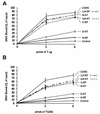
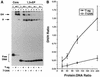
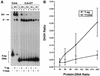
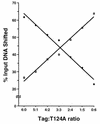
Cell cycle-dependent phosphorylation of simian virus 40 (SV40) large tumor antigen (T-ag) on threonine 124 is essential for the initiation of viral DNA replication. A T-ag molecule containing a Thr-->Ala substitution at this position (T124A) was previously shown to bind to the SV40 core origin but to be defective in DNA unwinding and initiation of DNA replication. However, exactly what step in the initiation process is defective as a result of the T124A mutation has not been established. Therefore, to better understand the control of SV40 replication, we have reinvestigated the assembly of T124A molecules on the SV40 origin. Herein it is demonstrated that hexamer formation is unaffected by the phosphorylation state of Thr 124. In contrast, T124A molecules are defective in double-hexamer assembly on subfragments of the core origin containing single assembly units. We also report that T124A molecules are inhibitors of T-ag double hexamer formation. These and related studies indicate that phosphorylation of T-ag on Thr 124 is a necessary step for completing the assembly of functional double hexamers on the SV40 origin. The implications of these studies for the cell cycle control of SV40 DNA replication are discussed.
Figures





References
-
- Bell S P, Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992;357:128–134. - PubMed
-
- Borowiec J A, Dean F B, Bullock P A, Hurwitz J. Binding and unwinding—how T antigen engages the SV40 origin of DNA replication. Cell. 1990;60:181–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

