Differential effect of zinc finger deletions on the binding of CTCF to the promoter of the amyloid precursor protein gene
- PMID: 10954607
- PMCID: PMC110710
- DOI: 10.1093/nar/28.17.3370
Differential effect of zinc finger deletions on the binding of CTCF to the promoter of the amyloid precursor protein gene
Abstract
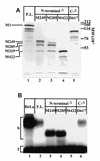
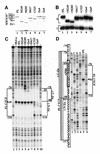
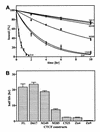
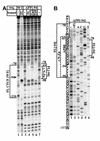
High levels of transcription from the amyloid precursor protein promoter are dependent on the binding of CTCF to the APBbeta core recognition sequence located between positions -82 and -93 upstream from the transcriptional start site. CTCF comprises 727 amino acids and contains 11 zinc finger motifs arranged in tandem that are flanked by 267 amino acids on the N-terminal side and 150 amino acids on the C-terminal side. Deletion of either the N- or the C-terminal regions outside of the zinc finger domain had no detrimental effect on the binding of CTCF to APBbeta. However, internal deletions of zinc fingers 5-7 completely abolished binding. The binding of full-length CTCF generated a DNase I protected domain extending from position -78 to -116, which was interrupted by a hypersensitive site at position -99. Selective deletions from the N- and C-terminal sides of the zinc finger domain showed that the N-terminal end of the zinc finger domain was aligned toward the transcriptional start site. Furthermore, deletions of zinc fingers peripheral to the essential zinc fingers 5-7 decreased the stability of the binding complex by interrupting sequence-specific interactions.
Figures



References
-
- Glenner G.G. and Wong,C.W. (1984) Biochem. Biophys. Res. Commun., 122, 1131–1135. - PubMed
-
- Mann D.M., Jones,D., Prinja,D. and Purkiss,M.S. (1990) Acta Neuropathol., 80, 318–327. - PubMed
-
- Kang J., Lemaire,H.G., Unterbeck,A., Salbaum,J.M., Masters,C.L., Grzeschik,K.H., Multhaup,G., Beyreuther,K. and Müller-Hill,B. (1987) Nature, 325, 733–736. - PubMed
-
- Golde T.E., Estus,S., Usiak,M., Younkin,L.H. and Younkin,S.G. (1990) Neuron, 4, 253–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

