Relative role of heme nitrosylation and beta-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation
- PMID: 10954746
- PMCID: PMC27634
- DOI: 10.1073/pnas.180155397
Relative role of heme nitrosylation and beta-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation
Abstract
To quantify the reactions of nitric oxide (NO) with hemoglobin under physiological conditions and to test models of NO transport on hemoglobin, we have developed an assay to measure NO-hemoglobin reaction products in normal volunteers, under basal conditions and during NO inhalation. NO inhalation markedly raised total nitrosylated hemoglobin levels, with a significant arterial-venous gradient, supporting a role for hemoglobin in the transport and delivery of NO. The predominant species accounting for this arterial-venous gradient is nitrosyl(heme)hemoglobin. NO breathing increases S-nitrosation of hemoglobin beta-chain cysteine 93, however only to a fraction of the level of nitrosyl(heme)hemoglobin and without a detectable arterial-venous gradient. A strong correlation between methemoglobin and plasma nitrate formation was observed, suggesting that NO metabolism is a primary physiological cause of hemoglobin oxidation. Our results demonstrate that NO-heme reaction pathways predominate in vivo, NO binding to heme groups is a rapidly reversible process, and S-nitrosohemoglobin formation is probably not a primary transport mechanism for NO but may facilitate NO release from heme.
Figures

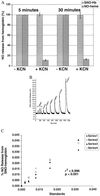
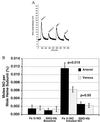

References
-
- Jia L, Bonaventura C, Bonaventura J, Stamler J S. Nature (London) 1996;380:221–226. - PubMed
-
- Stamler J S, Jia L, Eu J P, McMahon T J, Demchenko I T, Bonaventura J, Gernert K, Piantadosi C A. Science. 1997;276:2034–2037. - PubMed
-
- Gow A J, Stamler J S. Nature (London) 1998;391:169–173. - PubMed
-
- Bonaventura C, Ferruzzi G, Tesh S, Stevens R D. J Biol Chem. 1999;274:24742–24748. - PubMed
-
- Patel R P, Hogg N, Spencer N Y, Kalyanaraman B, Matalon S, Darley-Usmar V M. J Biol Chem. 1999;274:15487–15492. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

