Critical roles of glycosylphosphatidylinositol for Trypanosoma brucei
- PMID: 10954751
- PMCID: PMC27025
- DOI: 10.1073/pnas.180230697
Critical roles of glycosylphosphatidylinositol for Trypanosoma brucei
Abstract
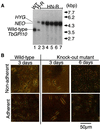
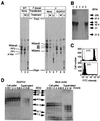
Trypanosoma brucei, the protozoan parasite responsible for sleeping sickness, evades the immune response of mammalian hosts and digestion in the gut of the insect vector by means of its coat proteins tethered to the cell surface via glycosylphosphatidylinositol (GPI) anchors. To evaluate the importance of GPI for parasite survival, we cloned and disrupted a trypanosomal gene, TbGPI10, involved in biosynthesis of GPI. TbGPI10 encodes a protein of 558 amino acids having 25% and 23% sequence identity to human PIG-B and Saccharomyces cerevisiae Gpi10p, respectively. TbGPI10 restored biosynthesis of GPI in a mouse mutant cell line defective in mouse Pig-b gene. TbGPI10 also rescued the inviability of GPI10-disrupted S. cerevisiae, indicating that TbGPI10 is the orthologue of PIG-B/GPI10 that is involved in the transfer of the third mannose to GPI. The bloodstream form of T. brucei could not lose TbGPI10; therefore, GPI synthesis is essential for growth of mammalian stage parasites. Procyclic form cells (insect stage parasites) lacking the surface coat proteins because of disruption of TbGPI10 are viable and grow slower than normal, provided that they are cultured in nonadherent flasks. In regular flasks, they adhered to the plastic surface and died. Infectivity to tsetse flies is partially impaired, particularly in the early stage. Therefore, parasitespecific inhibition of GPI biosynthesis should be an effective chemotherapy target against African trypanosomiasis.
Figures



Comment in
-
Glycosylphosphatidylinositol biosynthesis validated as a drug target for African sleeping sickness.Proc Natl Acad Sci U S A. 2000 Sep 26;97(20):10673-5. doi: 10.1073/pnas.97.20.10673. Proc Natl Acad Sci U S A. 2000. PMID: 11005849 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

