Low dose amiodarone and sotalol in the treatment of recurrent, symptomatic atrial fibrillation: a comparative, placebo controlled study
- PMID: 10956284
- PMCID: PMC1760955
- DOI: 10.1136/heart.84.3.251
Low dose amiodarone and sotalol in the treatment of recurrent, symptomatic atrial fibrillation: a comparative, placebo controlled study
Abstract
Objective: To assess and compare the safety and efficacy of amiodarone and sotalol in the treatment of patients with recurrent symptomatic atrial fibrillation.
Design: Prospective, randomised, single blind, placebo controlled study.
Setting: Tertiary cardiac referral centre.
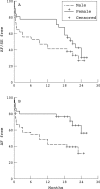
Patients: 186 consecutive patients (97 men, 89 women; mean (SD) age, 63 (10) years) with recurrent, symptomatic atrial fibrillation.
Interventions: 65 patients were randomised to amiodarone, 61 to sotalol, and 60 to placebo. Patients receiving amiodarone were maintained at a dose of 200 mg/day after a 30 day loading phase. The sotalol dose was 160-480 mg daily, as tolerated.
Main outcome measures: Recurrence of atrial fibrillation or side effects.
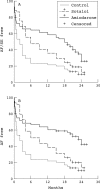
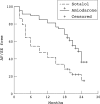
Results: In the amiodarone group, 31 of the 65 patients developed atrial fibrillation after an average of six months, while 15 (11 in sinus rhythm and four in atrial fibrillation) experienced significant side effects after an average of 16 months. In the sotalol group, relapse to atrial fibrillation occurred in 47 of the 61 patients after an average of eight months; three experienced side effects during the titration phase. In the placebo group, 53 of the 60 patients developed atrial fibrillation after an average of four months (p < 0.001 for amiodarone and sotalol v placebo; p < 0.001 for amiodarone v sotalol).
Conclusions: Both amiodarone and sotalol can be used for the maintenance of normal sinus rhythm in patients with symptomatic atrial fibrillation. Amiodarone is more effective but causes more side effects.
Figures
Comment in
- ACP J Club. 2001 Mar-Apr;134(2):53
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
