Yeast glycogen synthase kinase 3 is involved in protein degradation in cooperation with Bul1, Bul2, and Rsp5
- PMID: 10958669
- PMCID: PMC86186
- DOI: 10.1128/MCB.20.18.6712-6720.2000
Yeast glycogen synthase kinase 3 is involved in protein degradation in cooperation with Bul1, Bul2, and Rsp5
Abstract
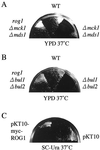
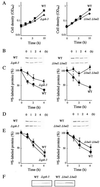
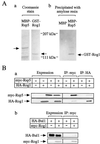
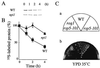
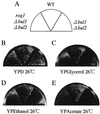
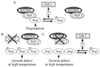
The yeast Saccharomyces cerevisiae has four genes, MCK1, MDS1 (RIM11), MRK1, and YOL128c, that encode glycogen synthase kinase 3 (GSK-3) homologs. The gsk-3 null mutant, in which these four genes are disrupted, shows temperature sensitivity, which is suppressed by the expression of mammalian GSK-3beta and by an osmotic stabilizer. Suppression of temperature sensitivity by an osmotic stabilizer is also observed in the bul1 bul2 double null mutant, and the temperature sensitivity of the bul1 bul2 double null mutant is suppressed by multiple copies of MCK1. We have screened rog mutants (revertants of gsk-3) which suppress the temperature sensitivity of the mck1 mds1 double null mutant and found that two of them, rog1 and rog2, also suppress the temperature sensitivity of the bul1 bul2 double null mutant. Bul1 and Bul2 have been reported to bind to Rsp5, a hect (for homologous to E6-associated-protein carboxyl terminus)-type ubiquitin ligase, but involvement of Bul1 and Bul2 in protein degradation has not been demonstrated. We find that Rog1, but not Rog2, is stabilized in the gsk-3 null and the bul1 bul2 double null mutants. Rog1 binds directly to Rsp5, and their interaction is dependent on GSK-3. Furthermore, Rog1 is stabilized in the npi1 mutant, in which RSP5 expression levels are reduced. These results suggest that yeast GSK-3 regulates the stability of Rog1 in cooperation with Bul1, Bul2, and Rsp5.
Figures



References
-
- Botstein D, Fallo S C, Stewart S E, Brennan M, Scherer S, Stinchcomb D T, Struhl K, Davis R W. Sterile host yeast (SHY); a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979;8:17–24. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
